Cisplatin-induced genetic alterations in KEAP1 promote therapeutic resistance in head and neck squamous cell carcinoma
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
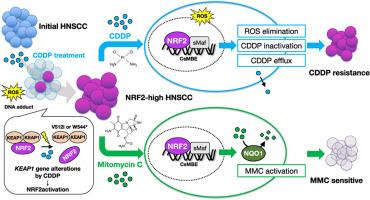
Cisplatin (CDDP) resistance remains a major challenge in the treatment of recurrent head and neck squamous cell carcinoma (HNSCC). The KEAP1-NRF2 system, a central regulator of cellular redox homeostasis, is frequently altered in cancer, but its contribution to acquired CDDP resistance in HNSCC remains to be clarified. To address this, we investigated NRF2 activation in CDDP resistance using seven parental (P) HNSCC cell lines and their CDDP-resistant (CR) derivatives. Among these P-CR pairs, three CR lines exhibited elevated NRF2 expression; two harbored KEAP1 mutations, and one had an NFE2L2 mutation that were present in both P and CR lines. These NRF2-high CR lines showed upregulation of NRF2 target genes and enrichment of xenobiotic metabolism and reactive oxygen species pathways. Mitomycin C (MMC), a cytotoxic agent for its synthetic lethality in NRF2-activated cancer cells, demonstrated strong cytotoxicity specifically in these NRF2-high CR lines. Immunohistochemical analysis on clinical samples found that high NRF2 expression was significantly associated with poor prognosis and was frequently observed in recurrent tumors following chemoradiotherapy with CDDP. These results suggest that CDDP therapy, while initially effective, may paradoxically promote tumor progression and therapeutic resistance by aberrantly activating the KEAP1-NRF2 axis. This redox-driven adaptation highlights a critical characteristic in NRF2-hyperactivated HNSCC that is exploitable by MMC treatment.
顺铂诱导的KEAP1基因改变促进头颈部鳞状细胞癌的治疗耐药
顺铂(CDDP)耐药性仍然是治疗复发性头颈部鳞状细胞癌(HNSCC)的主要挑战。KEAP1-NRF2系统是细胞氧化还原稳态的中心调节因子,在癌症中经常发生改变,但其在HNSCC中获得性CDDP耐药的作用尚不清楚。为了解决这个问题,我们使用7个亲本(P) HNSCC细胞系及其CDDP抗性(CR)衍生物研究了NRF2在CDDP抗性中的激活。在这些P-CR对中,3个CR系NRF2表达升高;其中两个携带KEAP1突变,一个携带NFE2L2突变,在P和CR系中都存在。NRF2高CR系NRF2靶基因上调,外源代谢和活性氧途径富集。丝裂霉素C (Mitomycin C, MMC)是一种对nrf2激活的癌细胞具有合成致死性的细胞毒性药物,在nrf2高CR细胞系中表现出很强的细胞毒性。临床样本免疫组化分析发现,NRF2高表达与预后不良显著相关,在CDDP放化疗后复发的肿瘤中屡见不鲜。这些结果表明,CDDP治疗虽然最初有效,但可能通过异常激活KEAP1-NRF2轴而矛盾地促进肿瘤进展和治疗耐药性。这种氧化还原驱动的适应强调了nrf2过度激活的HNSCC的一个关键特征,该特征可用于MMC治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


