Exendin-4 protects the dopaminergic neurons by attenuating inflammatory responses of microglial cells via activation of AMPK
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Parkinson's disease (PD) is a neurodegenerative disease characterized by preferential loss of the dopaminergic neurons in the substantia nigra and consequent occurrence of typical symptoms including resting tremor, rigidity and bradykinesia. PD remains a significant challenge due to the lack of disease-modifying drugs despite extensive research efforts. Glucagon-like peptide-1 and its analogues have shown neuroprotective properties. However, its specific molecular mechanisms for the neuroprotection remains to elucidate. In this study, we explored the anti-inflammatory and neuroprotective effects of exendin-4 by employing BV2 microglial cells and the MPTP-induced mouse model for PD. Our study showed that exendin-4 significantly suppressed the production of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6 in LPS-stimulated BV2 cells. Furthermore, exendin-4 increased AMPK phosphorylation and the activation of AMPK by exendin-4 played a crucial role in suppressing the production of proinflammatory cytokines, as addition of compound C, an inhibitor of AMPK diminished the effect. Additionally, exendin-4 demonstrated neuroprotective effects by attenuating SH-SY5Y cell death caused by conditioned media from BV2 cells exposed to LPS. Moreover, exendin-4 attenuated microglial and astroglial activation in the substantia nigra and striatum of MPTP-treated mice, which was accompanied by reduced proinflammatory cytokines. Exendin-4 also improved motor functions of MPTP-treated mice, as determined by beam test and rotarod test. In parallel with the anti-inflammatory effects, exendin-4 attenuated MPTP-mediated dopaminergic neurodegeneration as estimated by immunohistochemistry for tyrosine hydroxylase and HPLC analyses for the striatal dopamine and DOPAC. These results suggest that exendin-4 could be a promising therapeutic agent for PD, offering neuroprotection by modulating inflammatory pathways and preserving dopaminergic neurons.
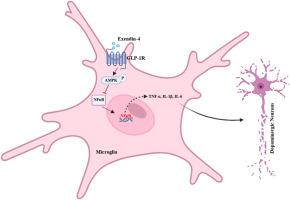
Exendin-4通过激活AMPK来减轻小胶质细胞的炎症反应,从而保护多巴胺能神经元
帕金森病(PD)是一种神经退行性疾病,其特征是黑质多巴胺能神经元优先丧失,随后出现静息性震颤、强直和运动迟缓等典型症状。尽管进行了广泛的研究,但由于缺乏改善疾病的药物,PD仍然是一个重大挑战。胰高血糖素样肽-1及其类似物具有神经保护作用。然而,其神经保护的具体分子机制尚不清楚。本研究通过BV2小胶质细胞和mptp诱导的PD小鼠模型,探讨exendin-4的抗炎和神经保护作用。我们的研究表明,exendin-4显著抑制lps刺激的BV2细胞中促炎细胞因子的产生,包括TNF-α、IL-1β和IL-6。此外,exendin-4增加了AMPK的磷酸化,exendin-4对AMPK的激活在抑制促炎细胞因子的产生中起着至关重要的作用,而添加化合物C(一种AMPK抑制剂)会减弱这一作用。此外,exendin-4通过降低暴露于LPS的BV2细胞的条件培养基引起的SH-SY5Y细胞死亡,显示出神经保护作用。此外,exendin-4降低了mptp处理小鼠黑质和纹状体中小胶质细胞和星形胶质细胞的激活,并伴有促炎细胞因子的减少。实验结果表明,Exendin-4还能改善mptp治疗小鼠的运动功能。在抗炎作用的同时,exendin-4通过酪氨酸羟化酶的免疫组化和纹状体多巴胺和多巴胺的高效液相色谱分析估计,可以减轻mptp介导的多巴胺能神经变性。这些结果表明exendin-4可能是一种很有前途的PD治疗剂,通过调节炎症通路和保存多巴胺能神经元提供神经保护。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuropharmacology
医学-神经科学
CiteScore
10.00
自引率
4.30%
发文量
288
审稿时长
45 days
期刊介绍:
Neuropharmacology publishes high quality, original research and review articles within the discipline of neuroscience, especially articles with a neuropharmacological component. However, papers within any area of neuroscience will be considered. The journal does not usually accept clinical research, although preclinical neuropharmacological studies in humans may be considered. The journal only considers submissions in which the chemical structures and compositions of experimental agents are readily available in the literature or disclosed by the authors in the submitted manuscript. Only in exceptional circumstances will natural products be considered, and then only if the preparation is well defined by scientific means. Neuropharmacology publishes articles of any length (original research and reviews).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


