Targeted inhibition of macrophage STING signaling alleviates inflammatory injury and ventricular remodeling in acute myocardial infarction
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Mitochondrial DNA (mtDNA) acts as a damage-associated molecular pattern to activate the stimulator of interferon genes (STING) signaling in macrophages, promoting tissue inflammation. However, its role in acute myocardial infarction (AMI) remains unclear. Macrophage-specific Sting1 knockout mice were used to validate STING's pathological role in AMI. Cardiac and liver mtDNA were used to activate macrophages in co-culture systems with cardiomyocytes to assess fibrosis and hypertrophy. Panaxatriol saponin (PTS) was tested for its ability to block mtDNA-driven macrophage activation and subsequent cardiomyocyte damage. STING–PTS binding ability was analyzed. AMI rats received PTS to evaluate its effects on myocardial inflammation and ventricular remodeling. In vivo, macrophage-specific Sting1 knockout reduced myocardial inflammation and injury after AMI. In vitro, mtDNA-activated macrophages induced cardiomyocyte fibrosis and hypertrophy through STING signaling. PTS suppressed mtDNA-driven macrophage activation by directly binding STING, thereby blocking inflammatory cascades. In AMI rats, PTS treatment attenuated acute inflammation and reversed ventricular remodeling. These findings establish the mtDNA–STING axis in macrophages as a critical driver of post-AMI inflammation and identify pharmacological STING inhibition with PTS as a promising therapeutic strategy. The study bridges genetic validation with translational applications, highlighting macrophage STING as a novel target for ischemic heart disease management.
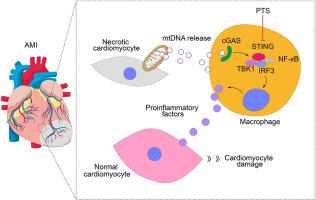
靶向抑制巨噬细胞STING信号可减轻急性心肌梗死的炎症损伤和心室重构
线粒体DNA (mtDNA)作为一种损伤相关的分子模式,激活巨噬细胞中的干扰素基因(STING)信号刺激因子,促进组织炎症。然而,其在急性心肌梗死(AMI)中的作用尚不清楚。利用巨噬细胞特异性敲除STING 1小鼠来验证STING在AMI中的病理作用。在与心肌细胞共培养的系统中,使用心脏和肝脏mtDNA激活巨噬细胞来评估纤维化和肥厚。我们测试了Panaxatriol皂苷(PTS)阻断mtdna驱动的巨噬细胞激活和随后的心肌细胞损伤的能力。分析STING-PTS的结合能力。急性心肌梗死大鼠接受PTS治疗,观察其对心肌炎症和心室重构的影响。在体内,巨噬细胞特异性敲除Sting1可减轻AMI后心肌炎症和损伤。体外,mtdna激活的巨噬细胞通过STING信号诱导心肌细胞纤维化和肥大。PTS通过直接结合STING抑制mtdna驱动的巨噬细胞活化,从而阻断炎症级联反应。在AMI大鼠中,PTS治疗减轻了急性炎症和逆转心室重构。这些发现确立了巨噬细胞中的mtDNA-STING轴是ami后炎症的关键驱动因素,并确定了PTS的药理STING抑制是一种有前景的治疗策略。该研究将基因验证与转化应用联系起来,突出了巨噬细胞STING作为缺血性心脏病治疗的新靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


