FtsZ as a novel target for antibiotics development: Promises and challenges
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Filamenting temperature-sensitive mutant Z (FtsZ), a protein essential for bacterial cell division, is highly conserved across bacterial species but absent in humans, positioning it as a strategic target for the development of antibiotics. Significant efforts to identify FtsZ inhibitors—via biochemical assays (e.g., GTPase activity) and cellular approaches (e.g., immunofluorescence)—have yielded over 100 natural products and synthetic compounds, whose cheminformatics clustering underscores a limited chemical diversity among the current scaffolds. Structural studies, including X-ray crystallography and cryo-electron microscopy, have resolved 97 FtsZ structures revealing conserved polymerization mechanisms and conformational plasticity, as exemplified by extremophile adaptations (e.g., Shewanella benthica from the high-pressure environment of the Mariana Trench's Challenger Deep). However, clinical translation is hindered by weak binding affinities, inhibitory inefficacy, dynamic conformational flexibility, and evolving drug resistance linked to FtsZ's functional plasticity. To address these challenges, future efforts should be directed to resolve transient assembly intermediates, leveraging machine learning with high-throughput screening, and integrating structural biology with pharmacokinetic optimization. Multidisciplinary strategies combining these approaches hold promise for translating FtsZ-focused research into clinically viable therapies, addressing the critical unmet need posed by antibiotics resistance.
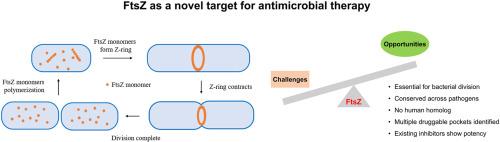
FtsZ作为抗生素开发的新靶点:前景与挑战
丝状温度敏感突变体Z (FtsZ)是细菌细胞分裂所必需的蛋白质,在细菌物种中高度保守,但在人类中不存在,将其定位为抗生素开发的战略靶点。通过生化分析(如GTPase活性)和细胞方法(如免疫荧光)鉴定FtsZ抑制剂的重大努力已经产生了100多种天然产物和合成化合物,其化学信息学聚类强调了当前支架中有限的化学多样性。结构研究,包括x射线晶体学和低温电子显微镜,已经解析了97个FtsZ结构,揭示了保守的聚合机制和构象可塑性,例如极端微生物的适应性(例如,来自马里亚纳海沟挑战者深处高压环境的底栖希瓦氏菌)。然而,与FtsZ的功能可塑性相关的弱结合亲和力、抑制无效、动态构象灵活性和不断发展的耐药性阻碍了临床翻译。为了应对这些挑战,未来的努力应针对解决瞬态组装中间体,利用机器学习与高通量筛选,并将结构生物学与药代动力学优化相结合。结合这些方法的多学科战略有望将ftsz重点研究转化为临床可行的治疗方法,解决抗生素耐药性带来的关键未满足需求。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


