Chemical knockdown of Keap1 and homoPROTAC-ing allergic rhinitis
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Allergic rhinitis (AR), a globally prevalent immune-mediated inflammatory condition, is still an incurable disease. In the present study, we have validated the impact of the Kelch-like ECH associated protein 1 (Keap1)-related oxidative stress and inflammatory response in clinical AR patient peripheral blood and nasal swab samples, emphasizing the biological relevance of Keap1 and AR. Targeting Keap1 -nuclear factor erythroid 2-related factor 2 (Nrf2) related anti-oxidative stress may be effective for AR intervention. Drawing inspiration from the Keap1 homodimerization and the E3 ligase characteristics, we herein present a design of novel bivalent molecules for chemical knockdown of Keap1. For the first time, we characterized ternary complexes of Keap1 dimer and one molecule of bivalent compounds. The best bivalent molecule 8 encompasses robust capacity to degrade Keap1 as a homoPROTACKEAP1. It efficaciously suppresses inflammatory cytokines in extensively different cells, including human nasal epithelial cells. Moreover, in an AR mouse model, we confirmed that the chemical degradation induced by homoPROTACKEAP1 led to therapeutic benefits in managing AR symptoms, oxidative stress and inflammation. In summary, our findings underscore the efficacy of targeting the Keap1 system through the homoPROTAC-ing technology as an innovative and promising treatment strategy for the incurable allergic disorders.
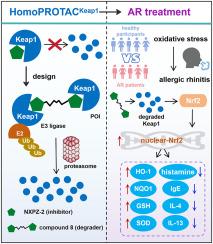
Keap1基因的化学表达下调与同质protac治疗变应性鼻炎的关系
过敏性鼻炎(AR)是一种全球普遍存在的免疫介导的炎症,目前仍是一种无法治愈的疾病。在本研究中,我们在临床AR患者外周血和鼻拭子样本中验证了kelch样ECH相关蛋白1 (Keap1)相关氧化应激和炎症反应的影响,强调了Keap1与AR的生物学相关性,靶向Keap1 -核因子-红细胞2相关因子2 (Nrf2)相关抗氧化应激可能有效干预AR。从Keap1同二聚化和E3连接酶的特性中获得灵感,我们设计了一种新的二价分子来化学敲除Keap1。首次表征了Keap1二聚体和一分子二价化合物的三元配合物。最好的二价分子8具有将Keap1降解为同源protackeap1的强大能力。它能有效抑制包括人鼻上皮细胞在内的多种不同细胞的炎症细胞因子。此外,在AR小鼠模型中,我们证实了由homoPROTACKEAP1诱导的化学降解在控制AR症状、氧化应激和炎症方面具有治疗益处。总之,我们的研究结果强调了通过同源protac技术靶向Keap1系统的有效性,作为一种创新的、有前景的治疗不可治愈的过敏性疾病的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


