Loss of Prominin 2 expression inhibits AKT/mTOR signaling to limit glycolysis and drive ferroptosis in breast cancer cells
IF 3.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular cell research
Pub Date : 2025-08-14
DOI:10.1016/j.bbamcr.2025.120047
引用次数: 0
Abstract
This study aimed to characterize the oncogenic functions of Prominin 2 (PROM2), the pro-cancer and ferroptosis resistance gene, in breast cancer (BC). PROM2 expression was analyzed using single-cell RNA sequencing and the TCGA database. Its expression was confirmed in BC tissues and cell lines using qRT-PCR, immunohistochemistry, and western blot assays. The effects of PROM2 were evaluated in vivo and in vitro. RNA sequencing and GSEA were used to investigate the potential underlying molecular mechanisms of PROM2 in BC. Co-immunoprecipitation was used to determine the interaction between AKT and PROM2. PROM2 expression was elevated in clinical samples and BC cells and positively correlated with a worse prognosis. Functional experiments demonstrated that PROM2 silencing suppressed tumor growth and malignancy. Mechanistically, PROM2 interacts with AKT to activate mTOR signaling, thereby promoting glycolysis and inhibiting ferroptosis. Specifically, for glycolysis, PROM2 silencing decreased glucose uptake, extracellular acidification rate, lactate production, and glycolysis-related enzyme expression, while increasing oxygen consumption. For ferroptosis, PROM2 silencing upregulated reactive oxygen species, malondialdehyde, iron, Fe2+, and downregulated SLC7A11, GPX4, and glutathione levels. Overexpression of AKT or the AKT agonist (SC79) reversed the effects of PROM2 silencing on BC cell glycolysis and ferroptosis. Our results suggest that PROM2 is an oncogenic gene that supports BC progression by enhancing glycolysis and inhibiting ferroptosis via AKT/mTOR signaling. Therefore, PROM2 may be a potential therapeutic target for BC treatment.
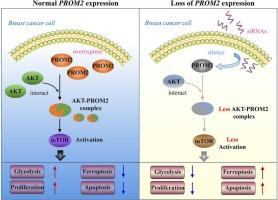
在乳腺癌细胞中,pronin2表达缺失可抑制AKT/mTOR信号通路限制糖酵解并驱动铁下垂。
本研究旨在探讨promein 2 (PROM2)在乳腺癌(BC)中的促癌和抗铁下垂基因的致癌功能。利用单细胞RNA测序和TCGA数据库分析PROM2的表达。通过qRT-PCR、免疫组织化学和western blot检测证实其在BC组织和细胞系中的表达。在体内和体外评价PROM2的作用。利用RNA测序和GSEA研究了PROM2在BC中的潜在分子机制。共免疫沉淀法测定AKT与PROM2的相互作用。PROM2在临床样本和BC细胞中的表达升高,并与较差的预后呈正相关。功能实验表明,PROM2沉默抑制肿瘤生长和恶性。机制上,PROM2与AKT相互作用激活mTOR信号,从而促进糖酵解,抑制铁下垂。具体来说,对于糖酵解,PROM2沉默降低了葡萄糖摄取、细胞外酸化速率、乳酸生成和糖酵解相关酶的表达,同时增加了氧气消耗。对于铁死亡,PROM2沉默上调活性氧、丙二醛、铁、Fe2+,下调SLC7A11、GPX4和谷胱甘肽水平。AKT或AKT激动剂(SC79)的过表达逆转了PROM2沉默对BC细胞糖酵解和铁凋亡的影响。我们的研究结果表明,PROM2是一种致癌基因,通过AKT/mTOR信号通路促进糖酵解和抑制铁凋亡,从而支持BC的进展。因此,PROM2可能是BC治疗的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.00
自引率
2.00%
发文量
151
审稿时长
44 days
期刊介绍:
BBA Molecular Cell Research focuses on understanding the mechanisms of cellular processes at the molecular level. These include aspects of cellular signaling, signal transduction, cell cycle, apoptosis, intracellular trafficking, secretory and endocytic pathways, biogenesis of cell organelles, cytoskeletal structures, cellular interactions, cell/tissue differentiation and cellular enzymology. Also included are studies at the interface between Cell Biology and Biophysics which apply for example novel imaging methods for characterizing cellular processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


