Experimental study on deep CBM well CO2 fracturing scaling:From microscale SEM observation to macroscale scale Inhibition—Ion polarization, thermal activation, and EDTA formulation
IF 4.6
0 ENERGY & FUELS
引用次数: 0
Abstract
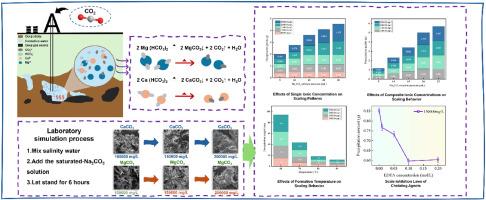
In the process of carbon dioxide dry fracturing of deep coalbed methane wells, serious scaling phenomenon occurred in the wellbore, but the wellbore scaling pattern are not clear, the scaling process was simulated indoors by adding saturated NaCO3 and NaHCO3 solutions into brines with different mineralisation levels and studied the chelating agent on the scaling process of the role of the law. The influence of formation water ion type, ion concentration, formation temperature, chelating agent EDTA on the amount of scaling and scale inhibition effect was investigated, and the change rule of scale crystals in the process of precipitation of carbonate ions with calcium and magnesium ions and the mode of action of scale inhibition were observed from the microscopic point of view. The results show that: ① the strength of cation polarity will change the scale crystal shape, Ca2+ polarization weak tend to form block CaCO3, Mg2+ polarization strong easy to generate needle MgCO3, both types of crystal deposits tend to aggregate.; ② the ion concentration in the formation is proportional to the amount of scaling. Mineralisation of 100000 mg/L, adding excess saturated Na2CO3 solution, the amount of scaling reached 1.084 g, mineralisation of 250000 mg/L, the amount of scaling reached 1.549 g, compared with the former increased by 42.90 %, the mineralisation increases through the solubility product breakthroughs and ionic strength effect significantly increases the amount of scaling, formation water mineralisation increased by 1 %, the amount of scaling increased by 0.286 % on average; ③ Temperature increases alter the state of ions by changing the activation energy. When the temperature reaches 80 °C, the shape of scale crystals changes from irregular spherical (calcium bicarbonate) to regular blocky (calcium carbonate). Compared with magnesium ions, calcium ions preferentially combine with anions to form scale. Under higher temperature conditions, the scale formed at the bottom of the well is mainly calcium carbonate. ④ Chelating agent EDTA can significantly reduce the scale formation by complexing Ca2+. In a system with a calcium ion concentration of 15,000 mg/L, the minimum scale inhibition rate is achieved by adding 20 mL of 0.1 mol/L EDTA. However, further increasing the dosage of the chelating agent will cause colloid flocculation and increase precipitation, leading to an increase in the scale formation.
煤层气井深部CO2压裂结垢实验研究:从微观尺度SEM观察到宏观尺度抑制-离子极化、热活化、EDTA配方
在深部煤层气井二氧化碳干法压裂过程中,井筒中出现了严重的结垢现象,但井筒结垢模式不清晰,通过在室内模拟结垢过程,在不同矿化水平的盐水中加入饱和的NaCO3和NaHCO3溶液,研究了螯合剂对结垢过程的作用规律。考察了地层水离子类型、离子浓度、地层温度、螯合剂EDTA对结垢量和阻垢效果的影响,并从微观角度观察了碳酸钙离子与钙、镁离子沉淀过程中结垢晶体的变化规律和阻垢作用方式。结果表明:①阳离子极性的强弱会改变晶体的尺度形状,Ca2+极化弱容易形成块状CaCO3, Mg2+极化强容易形成针状MgCO3,两种类型的晶体沉积都倾向于聚集;②地层中的离子浓度与结垢量成正比。矿化量为100000 mg/L时,加入过量饱和Na2CO3溶液,结垢量达到1.084 g,矿化量为250000 mg/L时,结垢量达到1.549 g,与前者相比增加了42.90%,矿化量通过溶解度产物突破和离子强度效应显著增加了结垢量,地层水矿化量增加了1%,结垢量平均增加了0.286%;温度升高通过改变活化能改变离子的状态。当温度达到80℃时,垢晶的形状由不规则的球形(碳酸氢钙)变为规则的块状(碳酸钙)。与镁离子相比,钙离子更倾向于与阴离子结合形成水垢。在较高温度条件下,井底形成的结垢主要是碳酸钙。④螯合剂EDTA能显著减少Ca2+络合形成的水垢。在钙离子浓度为15,000 mg/L的体系中,加入20 mL 0.1 mol/L的EDTA可达到最小阻垢率。然而,进一步增加螯合剂的用量会引起胶体絮凝,增加沉淀,导致结垢量增加。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


