Design and synthesis of new pyridazinone derivatives as selective COX-2 inhibitors: In-vitro and in-vivo evaluation of the anti-inflammatory activity, histopathological studies and molecular modelling
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
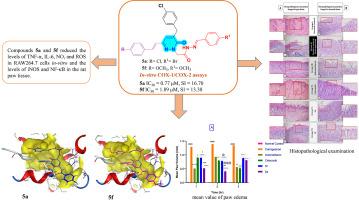
New pyridazinone derivatives 3a–d, 4a, 4b, and 5a–f were synthesized and tested for in-vitro inhibition of human COX-1 and COX-2. Compounds 5a and 5f showed strong COX-2 inhibition (IC50 = 0.77 and 1.89 μM; SI = 16.70 and 13.38) compared to indomethacin (IC50 = 0.42 μM, SI = 0.50) and celecoxib (IC50 = 0.35 μM, SI = 37.03). In LPS-induced RAW264.7 macrophages, ELISA results showed that compound 5a reduced TNF-α and IL-6 levels by 87 % and 76 %, outperforming celecoxib (67 % and 81 %), while compound 5f reduced them by 35 % and 32 %. RT-PCR revealed that compound 5a suppressed TNF-α and IL-6 mRNA by 82 % and 62 % (vs celecoxib 68 % and 70 %), whereas compound 5f achieved 27 % and 47 % reductions. Both compounds inhibited LPS-mediated NO by 35.7 % for compound 5a and 20 % for compound 5f and ROS production (compound 5a: 42 %, compound 5f: 21.3 %). In vivo, rat paw edema inhibition showed that both had strong anti-inflammatory effects, comparable to indomethacin and celecoxib, with a lower ulcer number and index compared to the indomethacin group. Gastric mucosal protection was 99.77 % for compound 5a and 83.08 % for compound 5f. Histopathology revealed paw tissue from treated groups had healthy epidermal layers with reduced inflammation. Stomach tissue from compound 5a-treated rats showed moderate tunica mucosa improvement and epithelial layer degeneration; compound 5f showed mild fundic mucosa improvement with inflammatory infiltration and mucosal desquamation. In paw tissue, both compounds reduced iNOS protein expression and significantly suppressed NF-κB. Molecular modelling indicated strong COX-2 binding affinities. ADME profiling confirmed drug-likeness: compound 5a fully complied with Veber's rules; compound 5f met Lipinski and Egan criteria without violations. Overall, compounds 5a and 5f demonstrate potent COX-2 selectivity, anti-inflammatory activity, reduced gastric toxicity, and favorable pharmacokinetics, positioning them as promising leads for safe and effective anti-inflammatory drug development.
设计和合成新的吡啶嗪酮衍生物作为选择性COX-2抑制剂:体外和体内抗炎活性评估,组织病理学研究和分子模型
合成新的吡嗪酮衍生物3a-d、4a、4b和5a-f,并测试其对人COX-1和COX-2的体外抑制作用。化合物5a和5f表现出较强的COX-2抑制作用(IC50分别为0.77和1.89 μM);与吲哚美辛(IC50 = 0.42 μM, SI = 0.50)和塞来昔布(IC50 = 0.35 μM, SI = 37.03)相比,SI分别为16.70和13.38。在lps诱导的RAW264.7巨噬细胞中,ELISA结果显示,化合物5a降低TNF-α和IL-6水平分别为87%和76%,优于塞来昔布(67%和81%),而化合物5f分别降低35%和32%。RT-PCR结果显示,化合物5a对TNF-α和IL-6 mRNA的抑制作用分别为82%和62%(塞来昔布分别为68%和70%),而化合物5f的抑制作用分别为27%和47%。两种化合物对化合物5a和化合物5f的抑制作用分别为35.7%和20%(化合物5a: 42%,化合物5f: 21.3%)。在体内,大鼠足部水肿抑制显示两者具有较强的抗炎作用,与吲哚美辛和塞来昔布相当,溃疡数量和指数低于吲哚美辛组。化合物5a对胃黏膜的保护作用为99.77%,化合物5f为83.08%。组织病理学显示,治疗组的爪组织表皮层健康,炎症减轻。复方5a处理大鼠胃组织出现中度粘膜改善和上皮变性;化合物5f表现为轻度基底粘膜改善,伴有炎症浸润和粘膜脱屑。在足跖组织中,两种化合物均可降低iNOS蛋白表达,并显著抑制NF-κB。分子模型表明其具有较强的COX-2结合亲和力。ADME分析证实药物相似:化合物5a完全符合Veber规则;化合物5f符合Lipinski和Egan标准,没有违规。总的来说,化合物5a和5f表现出强大的COX-2选择性,抗炎活性,降低胃毒性和良好的药代动力学,使它们成为安全有效的抗炎药物开发的有希望的先导物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


