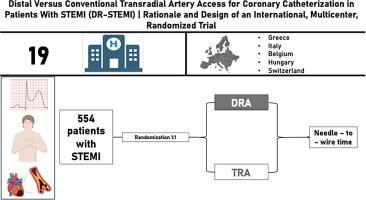
Distal versus conventional transradial artery access for coronary catheterization in patients with STEMI (DR-STEMI): Rationale and design of an international, multicenter, randomized trial
IF 3.5
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Rationale
Transradial access (TRA) constitutes the cornerstone for cardiac catheterization and is recommended by the multiple recent guidelines, irrespective of clinical presentation. The existing literature has evaluated distal transradial access (dTRA), as a feasible and safe approach in patients with chronic and acute coronary syndrome, excluding although patients presenting with ST- elevation myocardial infraction (STEMI).
Primary hypothesis
The current randomized clinical trial compares dTRA versus conventional TRA access in patients with STEMI undergoing coronary angiography and interventions regarding peri‑ and postprocedural characteristics.
Design
DR-STEMI is a prospective, open label, European, multicenter randomized-control trial which will include 554 patients (277 patients in each treatment arm). Patients with STEMI, will be screened on an all-comers basis for study inclusion and exclusion criteria, and those eligible will be allocated randomly (1:1), to dTRA versus TRA approach. The primary hypothesis of the study is that dTRA is noninferior to conventional TRA regarding the required time between the puncture of the radial artery and wire crossing of the infarct-related artery (i.e., needle-to-wire time).
Current status
Enrollment for the DR-STEMI trial began in May 2024, and as of April 15th, 2025, 309 patients have been enrolled in the study. Recruitment is expected to continue for approximately 12 months.
Trial registration
clinicaltrials.gov: NCT05605288
STEMI患者冠状动脉导管远端与传统经桡动脉通道(DR-STEMI):一项国际、多中心、随机试验的基本原理和设计
理由:不论临床表现如何,经桡骨通路(TRA)是心导管置入术的基石,是近期多个指南推荐的方法。现有文献已经评估了远端经桡骨通路(dTRA)作为慢性和急性冠状动脉综合征患者可行且安全的途径,尽管患者表现为ST段抬高心肌梗死(STEMI)。主要假设:目前的随机临床试验比较了dTRA与传统TRA在STEMI患者接受冠状动脉造影和干预手术前后的特征。DR-STEMI是一项前瞻性、开放标签、欧洲多中心随机对照试验,将纳入554例患者(每个治疗组277例)。STEMI患者将根据研究纳入和排除标准对所有患者进行筛选,符合条件的患者将随机(1:1)分配至dTRA和TRA方法。本研究的主要假设是,在桡动脉穿刺和梗死相关动脉导线穿越之间所需的时间(即针头到导线的时间)方面,dTRA不低于传统TRA。目前状态:DR-STEMI试验于2024年5月开始入组,截至2025年4月15日,已有309名患者入组。预计征聘工作将持续约12个月。试验注册:clinicaltrials.gov: NCT05605288。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

American heart journal
医学-心血管系统
CiteScore
8.20
自引率
2.10%
发文量
214
审稿时长
38 days
期刊介绍:
The American Heart Journal will consider for publication suitable articles on topics pertaining to the broad discipline of cardiovascular disease. Our goal is to provide the reader primary investigation, scholarly review, and opinion concerning the practice of cardiovascular medicine. We especially encourage submission of 3 types of reports that are not frequently seen in cardiovascular journals: negative clinical studies, reports on study designs, and studies involving the organization of medical care. The Journal does not accept individual case reports or original articles involving bench laboratory or animal research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


