Evaluation of the impact of a 8 week exposure to a portable light therapy device, Luminette®, on retinal function assessed by ElectroRetinoGraphy
IF 2.9
Q2 TOXICOLOGY
引用次数: 0
Abstract
Background
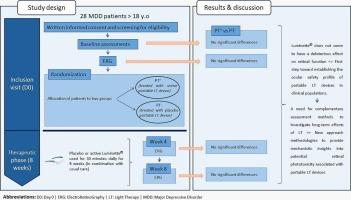
Major depressive disorder (MDD) treatment can be long and difficult to obtain. Thus, alternative non-pharmacological treatments, such as light therapy (LT), are increasingly recommended to treat MDD. For better treatment adherence, portable LT devices have been developed. However, more research is needed to better understand their safety of their use on patients’ physiology. One way to explore it is evaluating the retina function. That is why the aim of the study presented was to assess, thanks to Electroretinography (ERG), the impact on retinal function of an 8 weeks exposure to an active or a placebo LT portable device in MDD patients.
Method
MDD patients were treated with an active portable LT device or with a placebo LT device. The LT device tested was Luminette®. ERG measurements were carried out before the start of the LT treatment and 4 and 8 weeks afterwards.
Results
No significant differences were found in the ERG waveforms between the patients treated with active Luminette® and patients treated with the placebo device.
Conclusions
The use of the Luminette® device for 8 weeks, combined with usual care, did not result in any morphological or quantitative alterations in ERG waveforms in our cohort of MDD patients. This portable LT device would therefore be well tolerated at retinal level in MDD patients.
通过视网膜电图评估暴露于便携式光疗设备Luminette®8周对视网膜功能的影响
重度抑郁障碍(MDD)的治疗时间长且难以获得。因此,替代的非药物治疗,如光疗(LT),越来越多地被推荐用于治疗重度抑郁症。为了更好的治疗依从性,便携式LT设备已经被开发出来。然而,需要更多的研究来更好地了解它们对患者生理的安全性。一种探索的方法是评估视网膜功能。这就是为什么这项研究的目的是通过视网膜电图(ERG)来评估MDD患者暴露于活性或安慰剂LT便携式设备8周对视网膜功能的影响。方法对mdd患者采用主动便携式LT装置或安慰剂LT装置进行治疗。所测试的LT设备为Luminette®。在LT治疗开始前和治疗后4周和8周进行ERG测量。结果使用活性Luminette®治疗的患者与安慰剂治疗的患者的ERG波形无显著差异。结论:在我们的MDD患者队列中,使用Luminette®装置8周并结合常规护理,未导致任何形态学或定量的ERG波形改变。因此,这种便携式LT装置在MDD患者的视网膜水平上具有良好的耐受性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Toxicology
Environmental Science-Health, Toxicology and Mutagenesis
CiteScore
4.70
自引率
3.00%
发文量
33
审稿时长
82 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


