Abemaciclib impairs glioblastoma sphere formation by targeting the GSK3β-mediated transcriptional regulation of CD44 and TCF7L2
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Glioblastoma multiforme (GBM) is an aggressive brain tumor partly driven by cancer stem cells (CSCs). Abemaciclib demonstrates the potential for treating GBM, although its mechanisms beyond RB phosphorylation are not fully understood. This study reveals that Abemaciclib diminishes GBM sphere formation by influencing EMT pathways via GSK3β-mediated regulation of CD44 and TCF7L2. Treatment with Abemaciclib significantly hindered sphere formation in GBM cells, and transcriptomic analysis indicated EMT pathways suppression. Mechanistically, Abemaciclib consistently lowered the expression of CD44 and TCF7L2 in both parental and sphere cells by inhibiting GSK3β phosphorylation. A pharmacological GSK3β inhibitor produced similar effects, reinforcing the existence of a GSK3β-CD44/TCF7L2 axis. Moreover, orthotopic xenografts confirmed reduced tumor growth and CD44 expression in vivo. Analyses of TCGA and CGGA datasets revealed that the mesenchymal GBM subtype (MES-GBM), linked with poor outcomes, exhibits elevated EMT gene expression. Treatment of MES-like LN229 cells with Abemaciclib resulted in decreased phosphorylation of GSK3β and reductions in EMT-related gene expression. Our findings highlight a novel EMT-suppressive action of Abemaciclib, illustrating its therapeutic potential for targeting the CSCs and for treating the MES-GBM. This research provides mechanistic insights and justification for repurposing Abemaciclib as targeted therapies for aggressive glioblastoma.
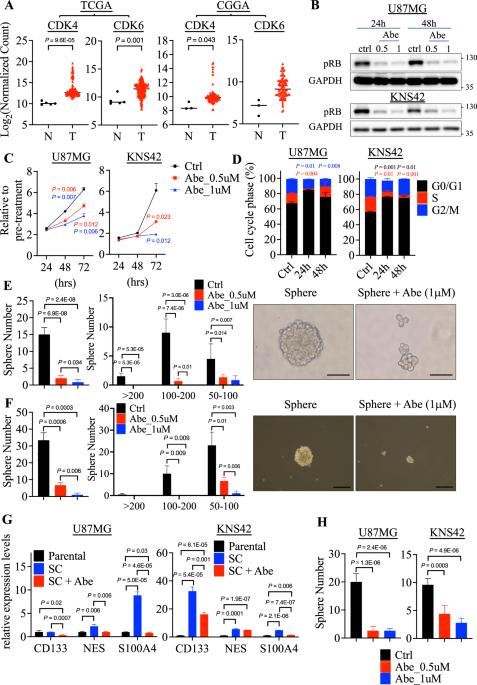
Abemaciclib通过靶向gsk3 β介导的CD44和TCF7L2的转录调控来损害胶质母细胞瘤球体的形成。
多形性胶质母细胞瘤(GBM)是一种侵袭性脑肿瘤,部分由癌症干细胞(CSCs)驱动。Abemaciclib显示了治疗GBM的潜力,尽管其RB磷酸化以外的机制尚不完全清楚。本研究表明,Abemaciclib通过gsk3 β介导的CD44和TCF7L2调控影响EMT通路,从而减少GBM球体的形成。Abemaciclib治疗显著阻碍了GBM细胞的球形形成,转录组学分析表明EMT通路受到抑制。机制上,Abemaciclib通过抑制GSK3β磷酸化持续降低亲本细胞和球细胞中CD44和TCF7L2的表达。一种药理GSK3β抑制剂产生类似的作用,强化了GSK3β- cd44 /TCF7L2轴的存在。此外,原位异种移植物在体内证实了肿瘤生长和CD44表达的减少。TCGA和CGGA数据集的分析显示,与预后不良相关的间质GBM亚型(MES-GBM)表现出EMT基因表达升高。用Abemaciclib处理mes样LN229细胞导致GSK3β磷酸化降低和emt相关基因表达降低。我们的研究结果强调了Abemaciclib的一种新的emt抑制作用,说明了其靶向CSCs和治疗MES-GBM的治疗潜力。这项研究为Abemaciclib作为侵袭性胶质母细胞瘤的靶向治疗提供了机制见解和理由。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


