Specific viral antibodies associate with anti-NMDAR encephalitis after herpes simplex encephalitis
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Herpes simplex encephalitis (HSE) patients may develop secondary anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis (NMDARE), associated with worsened long-term neurological outcome. Immunosuppressive treatment can limit NMDAR autoantibody-mediated pathology, but early predictive biomarkers for the risk of NMDARE are lacking. In a multicenter study, we performed unbiased antibody reactome profiling using Phage ImmunoPrecipitation Sequencing (PhIP-Seq). HSE patients with secondary NMDARE (n = 13) versus those without (n = 10) showed enhanced antibody responses against HSV-1, but not HSV-2, which comprised specific antibodies to five peptides of the HSV-1 UL42 and UL48 proteins. A score of these signature CSF antibodies identified HSE patients with secondary NMDARE with a sensitivity of 75%, a specificity of > 99%, a positive predictive value of 90%, a negative predictive value of > 97% and an odds ratio (OR) of 209 (CI: 28 – 1,582) across all individuals in this study, and with similar performance values in serum (>66%, >99%, >88%, >96%, OR 307 (15 – 6,089)). These signature antibodies represent a promising biomarker to identify HSE patients at risk for NMDARE development. In NMDARE patients without a history of HSE and in MS patients, no disease-associated HSV antibody reactivity patterns were detected. Furthermore, we introduced the Multiplexed Index Calculations of the Antibody Reactome (MICAR) metric to characterize proteomic targets of compartment-specific antibody responses, an approach that is applicable in neuroimmunology and other compartmentalized disease states.
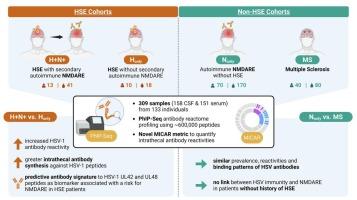
特异性病毒抗体与单纯疱疹脑炎后抗nmdar脑炎相关。
单纯疱疹脑炎(HSE)患者可发展继发性抗n -甲基- d -天冬氨酸受体(NMDAR)脑炎(NMDARE),并伴有长期神经预后恶化。免疫抑制治疗可以限制NMDAR自身抗体介导的病理,但缺乏NMDARE风险的早期预测性生物标志物。在一项多中心研究中,我们使用噬菌体免疫沉淀测序(PhIP-Seq)进行了无偏抗体反应组分析。继发性NMDARE的HSE患者(n = 13)与没有NMDARE的患者(n = 10)相比,对HSV-1的抗体反应增强,但对HSV-2的抗体反应没有增强,HSV-2包含针对HSV-1 UL42和UL48蛋白的五种肽的特异性抗体。这些签名的分数脑脊液抗体识别HSE患者二级NMDARE 75 %的敏感性,特异性的 > 99 %,90 %的阳性预测值,消极的预测价值的 > 97 %,比值比(或)209 (CI: 28 - 1582)在所有个人在这项研究中,在血清和类似的性能值(> 66 %,99 % >,> 88 % > 96 %,或307(15 - 6089))。这些特征抗体代表了一种有希望的生物标志物,可以识别有NMDARE发展风险的HSE患者。在没有HSE病史的NMDARE患者和MS患者中,未检测到与疾病相关的HSV抗体反应模式。此外,我们引入了抗体反应组(MICAR)指标的多重指数计算来表征室特异性抗体反应的蛋白质组学靶点,这种方法适用于神经免疫学和其他领域。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


