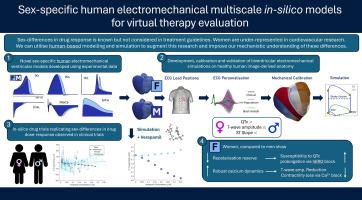
Sex-specific human electromechanical multiscale in-silico models for virtual therapy evaluation
IF 2.2
Journal of molecular and cellular cardiology plus
Pub Date : 2025-08-09
DOI:10.1016/j.jmccpl.2025.100479
引用次数: 0
Abstract
Background
Women are under-represented in cardiovascular research, leading to poorer outcomes. Investigating sex-differences in electromechanical function is essential for improving therapy evaluation. This study presents sex-specific human cellular and biventricular electromechanical models for mechanistic investigation of sex-differences in therapeutic response.
Methods
Protein genomic expression data from healthy human myocytes calibrated sex-specific electrophysiological models, integrated into biventricular models with male and female anatomies. Multi-scale validation utilised sex-specific clinical and experimental datasets, including responses to drug action. Ionic mechanisms underlying sex-differences in drug response were explored.
Results
Simulations showed agreement with clinical ECGs, including QTc intervals (Male: 312 ms; Female: 339 ms), and T-wave amplitude (6–9 % difference). Mechanical biomarkers (LVEF, Female: 68 %; Male: 50 %) matched sex-stratified UK Biobank data (n = 806; 46 % Male). ECG sex-characteristics were driven by ionic differences, while mechanical differences stemmed from anatomical and ionic differences. Simulations predicted exacerbated QTc prolongation under Dofetilide in women (54–78 % higher than males) and T-wave amplitude reduction in men (max: −0.25 mV). Verapamil increased T-wave amplitude in females and decreased it in males, without affecting QTc. Simulations demonstrated reduced repolarisation reserve and increased QTc susceptibility in women via hERG inhibition, while enhanced calcium buffering protected against T-wave amplitude loss. LVEF changes in response to calcium block were more sensitive to anatomical differences between male and female than to ionic sex phenotypes.
Conclusion
Sex differences in repolarisation reserve, calcium handling, and anatomy are key factors underpinning ECG and LVEF responses to drugs. Specifically, under calcium block, females showed more preserved LVEF, while under hERG block, females showed more QTc prolongation.
用于虚拟治疗评估的性别特异性人类机电多尺度计算机模型
女性在心血管研究中的代表性不足,导致较差的结果。研究机电功能的性别差异对改善治疗评价至关重要。本研究提出了性别特异性的人类细胞和双心室机电模型,用于治疗反应性别差异的机制研究。方法将健康人类肌细胞的蛋白质基因组表达数据整合到具有男性和女性解剖结构的双心室模型中,校准性别特异性电生理模型。多尺度验证利用了性别特异性临床和实验数据集,包括对药物作用的反应。探讨了药物反应性别差异的离子机制。结果模拟结果与临床心电图一致,包括QTc间隔(男性:312 ms;女性:339 ms),和t波振幅(6 - 9%的差异)。机械生物标志物(LVEF,女性:68%;男性:50%)匹配性别分层的UK Biobank数据(n = 806;46%男性)。ECG性别特征由离子差异驱动,而力学差异源于解剖和离子差异。模拟预测,服用多非利特后,女性QTc延长(比男性高54 - 78%),男性t波幅度降低(最大:- 0.25 mV)。维拉帕米增加了女性的t波振幅,降低了男性的t波振幅,但不影响QTc。模拟表明,通过hERG抑制,女性的重极化储备减少,QTc敏感性增加,而增强的钙缓冲可以防止t波振幅损失。LVEF对钙阻滞反应的变化对男女解剖差异比对离子性别表型更敏感。结论两性在复极储备、钙处理和解剖学上的差异是影响ECG和LVEF对药物反应的关键因素。其中,钙阻断下雌性LVEF保存较多,而hERG阻断下雌性QTc延长较多。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of molecular and cellular cardiology plus
Cardiology and Cardiovascular Medicine
自引率
0.00%
发文量
0
审稿时长
31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


