Proteomic and transcriptomic analyses reveal new insights into allergens in Ligustrum lucidum pollen
IF 2.8
2区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
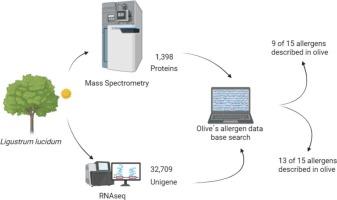
Pollen aeroallergens cause up to 40 % of respiratory allergies and are challenging to control due to their widespread distribution in the environment. The pollen of Ligustrum lucidum (privet) is a significant source of inhalant allergens. However, despite its clinical relevance, the protein composition of L. lucidum pollen remains poorly characterized. Therefore, we employed an integrated proteomic and transcriptomic approach to explore its potential allergen composition, focusing on possible cross-reactivity with Olea europea (olive), a well-studied allergenic relative. Using LC-MS/MS-based proteomics and RNA-seq transcriptomics, we detected 13 of the 15 known olive-like allergens, demonstrating high cross-species conservation. Proteomic analysis identified nine homologous allergens, including Ole e 1, Ole e 2, Ole e 3, Ole e 5, Ole e 6, Ole e 9, Ole e 12, Ole e 13, and Ole e 14. Transcriptomic analysis revealed four additional putative allergens: Ole e 8, Ole e 10, Ole e 11, and Ole e 15. These proteins shared 74–95 % sequence identity with their olive counterparts and exhibited multiple isoforms. Our findings provide a set of L. lucidum pollen potential allergens and highlight the utility of multi-omics in allergen discovery. However, further clinical validation of these putative novel allergens is needed to assess their role in sensitization and cross-reactivity.
Significance
Privet (Ligustrum), a genus within the Oleaceae family, is biologically significant due to its role in triggering allergic respiratory diseases worldwide. As a close relative of olive (Olea europaea) and ash (Fraxinus), privet shares allergenic proteins that contribute to cross-reactivity among sensitized individuals. Climate change has been shown to extend their flowering period, increasing pollen exposure and exacerbating allergic symptoms. Ligustrum is widely used in urban landscaping due to its rapid growth, resistance to pollution, and adaptability to diverse soil conditions, which facilitates its global spread across North America, Europe, Asia, and South America. Notably, L. lucidum is a major sensitizing agent in Mexico City, where 37 % of allergic patients react to its pollen. The first identified allergen, Lig v 1, shares homology with Ole e 1 and Fra e 1. At the same time, Lig v 2 (profilin) mirrors Ole e 2, highlighting the molecular basis for cross-reactivity within the Oleaceae family. Recent proteomic studies have uncovered additional allergens, including enolase, β-1,3-glucanase, and ATP synthase subunits, further elucidating privet's allergenic potential. The absence of genomic data for L. lucidum has hindered research; however, advances in transcriptomic and proteomic approaches have enabled the identification of 13 of 15 known olive-like allergens in privet pollen, paving the way for improved diagnostics and targeted therapies. This underscores the need for further investigation into Ligustrum's allergenic components, particularly as climate change and urbanization amplify its public health impact, as well as, the potential for improved diagnosis and targeted therapies.
蛋白质组学和转录组学分析揭示了女贞子花粉过敏原的新见解
花粉致敏原导致高达40%的呼吸道过敏,由于其在环境中的广泛分布,很难控制。女贞的花粉是吸入性过敏原的重要来源。然而,尽管其临床相关性,露珠l.l lucidum花粉的蛋白质组成特征仍然很差。因此,我们采用了综合的蛋白质组学和转录组学方法来探索其潜在的过敏原组成,重点是与橄榄树(Olea europea)可能的交叉反应性,橄榄树是一种已经得到充分研究的过敏原亲缘关系。利用LC-MS/MS-based蛋白质组学和RNA-seq转录组学,我们检测到15个已知橄榄样过敏原中的13个,显示出高度的跨物种保守性。蛋白质组学分析鉴定出9个同源过敏原,包括Ole e 1、Ole e 2、Ole e 3、Ole e 5、Ole e 6、Ole e 9、Ole e 12、Ole e 13和Ole e 14。转录组学分析显示了另外四种可能的过敏原:Ole e8、Ole e10、Ole e11和Ole e15。这些蛋白与橄榄蛋白具有74 - 95%的序列一致性,并表现出多种同工型。我们的发现提供了一组露珠菌花粉潜在的过敏原,并强调了多组学在过敏原发现中的应用。然而,这些假定的新型过敏原需要进一步的临床验证来评估它们在致敏和交叉反应中的作用。鸢尾属(Ligustrum)是油科植物中的一个属,因其在全球范围内引发过敏性呼吸道疾病而具有重要的生物学意义。作为橄榄(Olea europaea)和白蜡树(Fraxinus)的近亲,女贞具有致敏个体之间交叉反应的致敏蛋白。气候变化延长了它们的花期,增加了花粉暴露,加剧了过敏症状。女贞草因其生长迅速、抗污染、适应多种土壤条件而被广泛应用于城市园林绿化中,这使得其在北美、欧洲、亚洲和南美等地广泛传播。值得注意的是,L. lucidum是墨西哥城的主要致敏剂,其中37%的过敏患者对其花粉有反应。第一个发现的过敏原ligv1与Ole e1和frae1具有同源性。与此同时,lig2 (profilin)与ole2相对应,突出了油科植物交叉反应的分子基础。最近的蛋白质组学研究发现了其他过敏原,包括烯醇化酶、β-1,3-葡聚糖酶和ATP合成酶亚基,进一步阐明了女贞的致敏潜力。缺乏lucidum的基因组数据阻碍了研究;然而,转录组学和蛋白质组学方法的进步已经能够在女贞花粉中鉴定出15种已知橄榄样过敏原中的13种,为改进诊断和靶向治疗铺平了道路。这强调了进一步调查女贞子过敏成分的必要性,特别是在气候变化和城市化放大其公共卫生影响的情况下,以及改进诊断和靶向治疗的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of proteomics
生物-生化研究方法
CiteScore
7.10
自引率
3.00%
发文量
227
审稿时长
73 days
期刊介绍:
Journal of Proteomics is aimed at protein scientists and analytical chemists in the field of proteomics, biomarker discovery, protein analytics, plant proteomics, microbial and animal proteomics, human studies, tissue imaging by mass spectrometry, non-conventional and non-model organism proteomics, and protein bioinformatics. The journal welcomes papers in new and upcoming areas such as metabolomics, genomics, systems biology, toxicogenomics, pharmacoproteomics.
Journal of Proteomics unifies both fundamental scientists and clinicians, and includes translational research. Suggestions for reviews, webinars and thematic issues are welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


