NRF2-dependent suppression of selenoprotein P expression promotes intracellular selenium metabolic remodeling and upregulation of antioxidant selenoproteins in hepatocellular carcinoma
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Selenium-containing antioxidant enzymes such as glutathione peroxidase 4 (GPx4) and thioredoxin reductase 1 (TrxR1, encoded by TXNRD1) have emerged as therapeutic targets in hepatocellular carcinoma (HCC), a highly treatment-resistant cancer. Hepatocytes play a central role in selenium metabolism by synthesizing and secreting selenoprotein P (SeP, encoded by SELENOP), the major selenium containing protein in plasma, which supplies selenium to peripheral tissues. Although decreased circulating SeP levels have been associated with HCC progression and poor prognosis, the underlying mechanisms remain unclear.
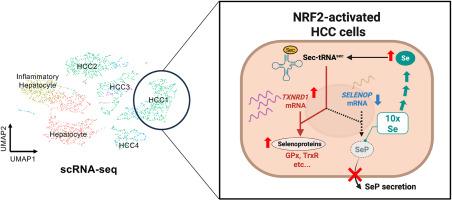
In this study, we reanalyzed publicly available single-cell RNA sequence data of HCC tumors and identified a distinct tumor cell cluster characterized by reduced SELENOP expression, enhanced GPX4 and TXNRD1 expression, and activation of NRF2 signaling. In HepG2 cells, pharmacological and genetic activation of NRF2 suppressed SeP expression, elevated TrxR1 levels, and promoted intracellular selenium accumulation. Consistently, SeP knockout (KO) cells exhibited increased intracellular selenium, upregulation of GPx1 and GPx4, and resistance to ferroptosis. Similarly, under selenium-deficient dietary conditions, SeP KO mice showed elevated hepatic selenium and GPx1 expression compared to wild-type controls.
These findings uncover a novel NRF2-mediated selenium metabolic remodeling mechanism in HCC, in which SeP suppression promotes intracellular selenium retention and selective upregulation of antioxidant selenoproteins. This redox adaptation contributes to ferroptosis resistance and may represent a potential therapeutic axis in liver cancer.
nrf2依赖性硒蛋白P表达抑制促进肝癌细胞内硒代谢重塑和抗氧化硒蛋白上调
含硒抗氧化酶如谷胱甘肽过氧化物酶4 (GPx4)和硫氧还蛋白还原酶1 (TrxR1,由TXNRD1编码)已成为治疗肝细胞癌(HCC)的靶点。肝细胞通过合成和分泌硒蛋白P (SELENOP编码的硒蛋白)在硒代谢中发挥核心作用,硒蛋白P是血浆中主要的含硒蛋白,向外周组织提供硒。虽然循环SeP水平降低与HCC进展和预后不良有关,但其潜在机制尚不清楚。在这项研究中,我们重新分析了公开的肝癌肿瘤单细胞RNA序列数据,并确定了一个独特的肿瘤细胞群,其特征是SELENOP表达降低,GPX4和TXNRD1表达增强,NRF2信号激活。在HepG2细胞中,NRF2的药理和遗传激活抑制SeP表达,升高TrxR1水平,促进细胞内硒的积累。一致地,SeP敲除(KO)细胞表现出细胞内硒增加,GPx1和GPx4上调,以及对铁凋亡的抗性。同样,在缺硒饮食条件下,SeP KO小鼠与野生型对照相比,肝脏硒和GPx1表达升高。这些发现揭示了一种新的nrf2介导的HCC硒代谢重塑机制,其中SeP抑制促进细胞内硒保留和抗氧化硒蛋白的选择性上调。这种氧化还原适应有助于铁下垂抵抗,并可能代表肝癌的潜在治疗轴。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


