Toxic effects of trichlorfon on the hepatopancreas of Chinese mitten crab (Eriocheir sinensis): Tissue damage, oxidative stress, and Ferroptosis
IF 2.2
2区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology D-Genomics & Proteomics
Pub Date : 2025-08-11
DOI:10.1016/j.cbd.2025.101601
引用次数: 0
Abstract
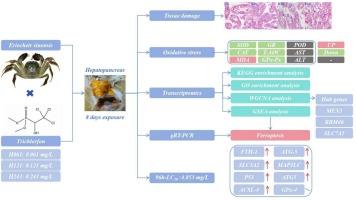
This study investigated the toxic effects and mechanisms of trichlorfon (TCF) on the hepatopancreas of Chinese mitten crabs (Eriocheir sinensis). The acute toxicity test results showed that the 96 h-LC50 of TCF on E. sinensis was 4.853 mg/L. We conducted an 8-day short-term exposure experiment with TCF concentrations of 0, 0.061, 0.121, and 0.243 mg/L. The results showed that TCF exposure induces oxidative stress and causes tissue damage and lesions to the hepatopancreas, including hepatic tubules exhibiting disruption, swelling, necrosis, and an ill-defined structure. Transcriptomics revealed differentially expressed genes (DEGs) at the different concentrations, KEGG, and GO enrichment analysis showed that the DEGs were mainly involved in the bile secretion pathway and iron ion binding terms. GSEA was used for single-function enrichment analysis of cell growth and death. We found that DEGs at TCF concentrations of 0.121 and 0.243 mg/L were significantly enriched in the Ferroptosis pathway. Moreover, the synaptic vesicle cycle, GABAergic synapse, and serotonergic synapse were significantly enriched in DEGs at 0.243 mg/L, which indicated that TCF may pose a potential threat to the nervous system. The qPCR detection of Ferroptosis-related genes demonstrated that TCF induces hepatopancreatic Ferroptosis in E. sinensis. We hypothesized that lysosomal autophagy, mainly induced through FTH1 and NCOA4 interactions, released ferric ions, thereby indirectly contributing to Ferroptosis. In addition, WGCNA identified two key modules associated with the phenotype, and further identified the core hub genes were MEX3, RBM46, SLC7A5, and TYR. The results of this study can provide insights into the toxic effects of TCF on aquatic crustaceans.
敌百虫对中华绒螯蟹肝胰脏的毒性作用:组织损伤、氧化应激和铁下垂
本研究探讨了敌百虫对中华绒螯蟹肝胰腺的毒性作用及其机制。急性毒性试验结果表明,TCF对中华依蚊的96 h-LC50为4.853 mg/L。我们对TCF浓度分别为0、0.061、0.121和0.243 mg/L进行了为期8天的短期暴露实验。结果表明,TCF暴露诱导氧化应激,导致肝胰腺组织损伤和病变,包括肝小管出现破坏、肿胀、坏死和结构不清。转录组学揭示了不同浓度下的差异表达基因(DEGs), KEGG和GO富集分析显示,这些差异表达基因主要参与胆汁分泌途径和铁离子结合项。GSEA用于细胞生长和死亡的单功能富集分析。我们发现,TCF浓度为0.121和0.243 mg/L时,DEGs在铁下垂途径中显著富集。此外,在0.243 mg/L时,突触囊泡周期、gaba能突触和5 -羟色胺能突触的DEGs显著富集,表明TCF可能对神经系统构成潜在威胁。通过对嗜铁相关基因的qPCR检测表明,TCF可诱导中华赤霉素肝胰腺嗜铁中毒。我们假设溶酶体自噬主要通过FTH1和NCOA4相互作用诱导,释放铁离子,从而间接导致铁凋亡。此外,WGCNA鉴定出与表型相关的两个关键模块,并进一步鉴定出核心枢纽基因为MEX3、RBM46、SLC7A5和TYR。本研究的结果可以为TCF对水生甲壳类动物的毒性作用提供见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.10
自引率
3.30%
发文量
69
审稿时长
33 days
期刊介绍:
Comparative Biochemistry & Physiology (CBP) publishes papers in comparative, environmental and evolutionary physiology.
Part D: Genomics and Proteomics (CBPD), focuses on “omics” approaches to physiology, including comparative and functional genomics, metagenomics, transcriptomics, proteomics, metabolomics, and lipidomics. Most studies employ “omics” and/or system biology to test specific hypotheses about molecular and biochemical mechanisms underlying physiological responses to the environment. We encourage papers that address fundamental questions in comparative physiology and biochemistry rather than studies with a focus that is purely technical, methodological or descriptive in nature.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


