Steering charge dynamics and surface reactivity for photocatalytic selective methane oxidation to ethane over Au/Ti-CeO2
IF 13.5
2区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The selective oxidation of methane to value-added chemicals under mild conditions presents a sustainable yet challenging route, hindered by sluggish CH4 activation and overoxidation. Herein, we report a delicate strategy combining Ti doping and Au loading to construct a high-performance Au/Ti-CeO2 photocatalyst for ethane production from oxidative methane coupling. The optimized catalyst achieves a C2H6 production rate of 2971.4 μmol g−1 h−1 with 85.1 % C2+ selectivity, stably operating over 20 reaction cycles. In situ X-ray photoelectron spectroscopy, electron paramagnetic resonance, and diffuse reflectance infrared Fourier transform spectroscopy analyses reveal that Ti doping introduces impurity energy levels into CeO2, promoting directional electron migration to surface Au nanoparticles (NPs) via a built-in electric field. The Au NPs act as electron accumulation sites to activate O2, facilitate ∗CH3 radical coupling into C2H6, and stabilize reactive intermediates, thus enhancing charge separation and suppressing intermediate overoxidation. This study highlights the significance of synergistic modulation via elemental doping and cocatalyst engineering in tuning charge dynamics and surface reactivity for efficient photocatalytic methane conversion.
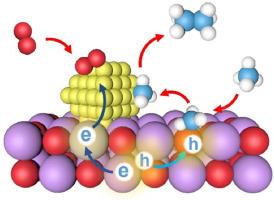
Au/Ti-CeO2光催化选择性甲烷氧化制乙烷的转向电荷动力学和表面反应性
甲烷在温和条件下选择性氧化生成增值化学品是一条可持续但具有挑战性的途径,受到CH4激活缓慢和过度氧化的阻碍。本文报道了一种结合Ti掺杂和Au负载的精细策略,构建了一种用于氧化甲烷偶联生产乙烷的高性能Au/Ti- ceo2光催化剂。优化后的催化剂C2H6产率为2971.4 μmol g−1 h−1,C2+选择性为85.1%,可稳定运行20个反应周期。原位x射线光电子能谱、电子顺磁共振和漫反射红外傅立叶变换能谱分析表明,Ti掺杂在CeO2中引入了杂质能级,通过内置电场促进了电子向表面金纳米粒子(NPs)的定向迁移。Au NPs作为电子积累位点激活O2,促进∗CH3自由基偶联到C2H6,稳定活性中间体,从而促进电荷分离和抑制中间体过度氧化。本研究强调了通过元素掺杂和助催化剂工程进行协同调制在调整电荷动力学和表面反应性以实现高效光催化甲烷转化方面的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


