Species-specific serine metabolism differentially controls natural killer cell functions
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
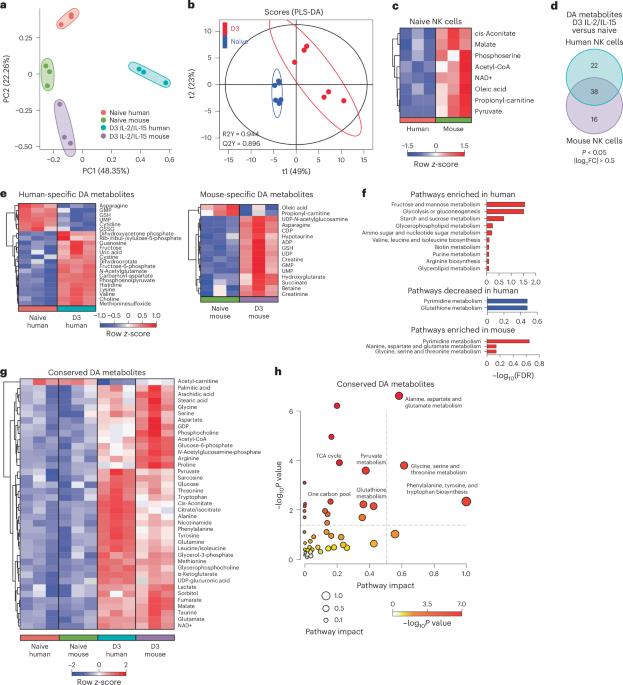
Immune cells undergo rapid metabolic reprogramming to fuel effector responses. However, whether the metabolic pathways that supply these functions differ between human and mouse immune cells is poorly understood. Using a comparative metabolomics approach, here we show both conserved and species-distinct metabolite alterations in cytokine-activated primary human and mouse natural killer (NK) cells. Activated human NK cells fail to perform de novo serine synthesis, resulting in broadly impaired effector functions when serine starved ex vivo or during in vivo dietary serine restriction, limiting their antitumour function. In contrast, activated mouse NK cells perform de novo serine synthesis to fuel one-carbon metabolism and proliferation, resulting in increased metabolic flexibility during ex vivo and dietary serine restriction. While NK cells from both species require one-carbon metabolism to proliferate and produce interferon-γ, GCLC-dependent glutathione synthesis tunes cytotoxic versus inflammatory function in human NK cells. Thus, activated NK cell functions display species-specific requirements for serine metabolism, and environmental serine availability dictates activated human NK cell functions. Li et al. characterize overlapping and divergent metabolic requirements of human and murine NK cells in response to activation, with a focus on serine metabolism
物种特异性丝氨酸代谢差异控制自然杀伤细胞功能
免疫细胞经历快速的代谢重编程以产生燃料效应反应。然而,提供这些功能的代谢途径在人类和小鼠免疫细胞之间是否不同,人们知之甚少。使用比较代谢组学方法,在这里,我们显示了细胞因子激活的原代人和小鼠自然杀伤(NK)细胞中保守的和物种不同的代谢物改变。激活的人类NK细胞不能进行从头合成丝氨酸,导致在体外缺乏丝氨酸或体内限制膳食丝氨酸时,效应细胞功能广泛受损,从而限制了它们的抗肿瘤功能。相比之下,激活的小鼠NK细胞通过从头合成丝氨酸来促进单碳代谢和增殖,从而在离体和饮食丝氨酸限制期间增加代谢灵活性。尽管两种物种的NK细胞都需要单碳代谢来增殖和产生干扰素-γ,但gclc依赖性谷胱甘肽合成调节了人类NK细胞的细胞毒性和炎症功能。因此,活化的NK细胞功能显示了丝氨酸代谢的物种特异性需求,而环境丝氨酸的可用性决定了活化的人类NK细胞功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


