Immune evasion from macrophages by NEAT1-induced CD24 in liver cancer
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The limited efficacy of immune checkpoint inhibitors in hepatocellular carcinoma (HCC) can arise from the involvement of immunosuppressive cells, such as macrophages. In the present study, we found that the long noncoding RNA NEAT1, particularly its short isoform (NEAT1v1) induced the expression of CD24, which is known as an immune checkpoint molecule for macrophages. Mechanistically, NEAT1v1 sponged miR-320a-3p to upregulate the transcription factor SP1, which in turn, activated CD24 transcription. A spheroid co-culture of primary or THP-1-derived macrophages with HCC cells revealed that NEAT1v1 suppressed M1 marker expression and phagocytic activity in macrophages. In a syngeneic subcutaneous model of HCC, Neat1v1 increased the tumor infiltration of M2-like macrophages and induced resistance to anti-Pd-1 antibody, while combination of the anti-Pd-1 and anti-Cd24 antibodies significantly suppressed the tumor growth. Finally, NEAT1, SP1, and CD24 expression increased in tumor tissues from patients with HCC, compared to adjacent normal tissues, whereas miR-320a-3p was significantly downregulated. Moreover, plasma NEAT1 cell-free RNA was significantly decreased after therapeutic intervention. Taken together, NEAT1v1 protects HCC cells from macrophages by sending a “Don’t Eat Me” signal via CD24, and is therefore, a potential target molecule for the treatment and diagnosis of HCC.
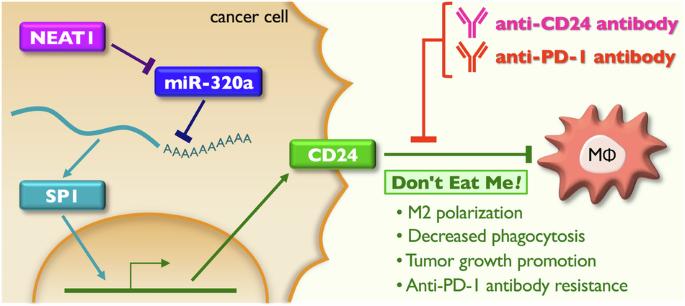
neat1诱导的CD24对肝癌巨噬细胞的免疫逃避。
免疫检查点抑制剂在肝细胞癌(HCC)中的有限疗效可能源于免疫抑制细胞(如巨噬细胞)的参与。在本研究中,我们发现长链非编码RNA NEAT1,特别是其短亚型NEAT1v1,诱导巨噬细胞免疫检查点分子CD24的表达。在机制上,NEAT1v1海绵miR-320a-3p上调转录因子SP1,进而激活CD24转录。原代或thp -1来源的巨噬细胞与HCC细胞共培养发现NEAT1v1抑制了巨噬细胞中M1标记物的表达和吞噬活性。在同基因肝癌皮下模型中,Neat1v1增加了m2样巨噬细胞的肿瘤浸润,诱导抗pd -1抗体的耐药,而抗pd -1和抗cd24抗体联合使用可显著抑制肿瘤生长。最后,与邻近正常组织相比,HCC患者肿瘤组织中NEAT1、SP1和CD24的表达增加,而miR-320a-3p则显著下调。此外,治疗干预后血浆NEAT1无细胞RNA显著减少。综上所述,NEAT1v1通过CD24发送“不要吃我”信号来保护HCC细胞免受巨噬细胞的侵袭,因此是HCC治疗和诊断的潜在靶标分子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


