Beta-blocker side-effects in clinical practice: A nationwide approach
IF 3.5
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background
Concerns regarding side-effects of beta-blockers (BBs) are frequent but data regarding the incidence of side-effects are conflicting and real-world data are sparse. Hence, we aimed to investigate the absolute and relative risks of BB side-effects in clinical practice.
Methods
Using Danish nationwide registers, we included Danish hypertensive patients initiating antihypertensive treatment with a BB or calcium-channel blocker (CCB). We computed crude as well as standardized 1-year risks and adjusted risk ratios of BB side-effects (depression, anxiety/insomnia, gastrointestinal side-effects, erectile dysfunction, and dizziness/fainting) compared with CCB treatment.
Results
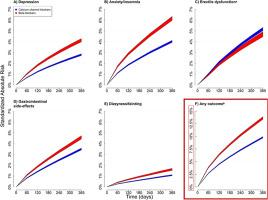
We included 64,722 patients initiating treatment with a BB and 181,880 patients initiating treatment with a CCB. In patients initiated on BB, the standardized 1-year risk of any outcome, erectile dysfunction exempt, was 13.7% (95% CI: 13.4%-13.9%). The 1-year risk of specific BB side-effects was the highest for anxiety/insomnia (6.2%, 95% CI: 6.0%-6.3%), gastrointestinal side-effects (4.6%, 95% CI: 4.4%-4.7%), and erectile dysfunction (4.7%, 95% CI: 4.5%-4.9%).
The risk of side-effects was consistently increased when comparing BB treatment with CCBs including depression (Risk Ratio [RR] 1.48, 95% CI 1.41-1.55), anxiety/insomnia (RR 1.53, 95% CI 1.47-1.59), gastrointestinal side-effects (1.31, 95% CI 1.25-1.36), and dizziness/fainting (RR 1.50, 95% CI 1.38-1.61), but not erectile dysfunction (RR 0.91, 95% CI, 0.85-0.96).
Conclusions
In a large nationwide cohort, the incidence of BB side-effects was clinically relevant and consistently increased compared with CCBs with the exception of erectile dysfunction, which carried similar risks for both treatments.
-受体阻滞剂在临床实践中的副作用:一项全国性的方法。
背景:对-受体阻滞剂(BBs)副作用的关注是频繁的,但关于副作用发生率的数据是相互矛盾的,真实世界的数据很少。因此,我们的目的是调查临床实践中BB副作用的绝对和相对风险。方法:使用丹麦全国范围的登记资料,我们纳入了开始使用BB或钙通道阻滞剂(CCB)进行抗高血压治疗的丹麦高血压患者。我们计算了与CCB治疗相比,BB副作用(抑郁、焦虑/失眠、胃肠道副作用、勃起功能障碍和头晕/昏厥)的1年风险和标准化风险,并调整了风险比。结果:我们纳入了64,722例开始接受BB治疗的患者和181,880例开始接受CCB治疗的患者。在开始接受BB治疗的患者中,除勃起功能障碍外,任何结果的标准化一年风险为13.7% (95% CI: 13.4%-13.9%)。特异性BB副作用的一年风险最高的是焦虑/失眠(6.2%,95% CI: 6.0%-6.3%)、胃肠道副作用(4.6%,95% CI: 4.4%-4.7%)和勃起功能障碍(4.7%,95% CI: 4.5%-4.9%)。与CCBs相比,BB治疗的副作用风险持续增加,包括抑郁(风险比[RR] 1.48, 95% CI 1.41-1.55)、焦虑/失眠(RR 1.53, 95% CI 1.47-1.59)、胃肠道副作用(1.31,95% CI 1.25-1.36)和头晕/昏厥(RR 1.50, 95% CI 1.38-1.61),但没有勃起功能障碍(RR 0.91, 95% CI 0.85-0.96)。结论:在一项全国性的大型队列研究中,除勃起功能障碍外,与CCBs相比,BB副作用的发生率与临床相关且持续增加,两种治疗的风险相似。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

American heart journal
医学-心血管系统
CiteScore
8.20
自引率
2.10%
发文量
214
审稿时长
38 days
期刊介绍:
The American Heart Journal will consider for publication suitable articles on topics pertaining to the broad discipline of cardiovascular disease. Our goal is to provide the reader primary investigation, scholarly review, and opinion concerning the practice of cardiovascular medicine. We especially encourage submission of 3 types of reports that are not frequently seen in cardiovascular journals: negative clinical studies, reports on study designs, and studies involving the organization of medical care. The Journal does not accept individual case reports or original articles involving bench laboratory or animal research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


