Geochemical associations of metals in corrosion scales from lead and copper drinking water pipes: Assessing the dissolution and bioaccessibility potentials
IF 3.4
3区 地球科学
Q1 GEOCHEMISTRY & GEOPHYSICS
引用次数: 0
Abstract
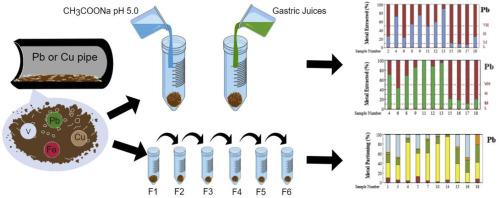
This study investigated the speciation of metals accumulated in corrosion scales within drinking water distribution pipes by comparing two methods: X-ray diffraction (XRD) and an operationally defined chemical sequential extraction technique. Additionally, two single-step chemical extraction methods were employed to target metal fractions that are considered potentially bio-accessible if scale particulates are ingested through tap water consumption. The first is a 1.0 M CH3COONa solution adjusted to pH 5 with CH3COOH to target metal fractions operationally defined as exchangeable and bound to carbonate minerals. The second is a solution mimicking gastric juice (USEPA Method 1340). The corrosion scales analyzed were obtained from lead (Pb) and copper (Cu) drinking water pipes excavated from U.S. municipalities, where the metal concentrations released into the water are expected to meet the thresholds established by the Lead and Copper Rule (LCR). Although the amorphous phases were not detectable by XRD, our findings highlighted their potential contribution to the measured concentrations of dissolved metals, as inferred from the high concentrations of chemically extracted metals that were not associated with the minerals identified in the diffractograms. Therefore, identifying crystalline minerals using XRD alone is insufficient for predicting the potential of corrosion scales to contaminate potable water flowing through municipal distribution pipes with metals that pose human health risks. The assessment of potentially bio-accessible metal fractions showed that for Pb, Cu, and to some extent zinc (Zn), extraction with CH3COONa resulted in dissolved metal concentration trends similar to those obtained with the US-EPA Method 1340, although the correlations were not strong. Overall, the US-EPA Method 1340 dissolved significantly higher amounts of Pb, Cu, Mn, Fe, Cr, and Sn compared to the CH3COONa method, suggesting that these methods, if assumed acceptable for predicting the pool of bio-accessible metals, cannot be used interchangeably for corrosion scales.
铅和铜饮用水管道腐蚀鳞片中金属的地球化学关联:评估溶解和生物可及性电位
本研究通过比较x射线衍射(XRD)和可操作定义的化学顺序萃取技术两种方法,研究了饮用水配水管腐蚀垢中积累的金属的形态。此外,两种单步化学萃取方法被用于目标金属组分,如果水垢颗粒通过自来水摄入,则被认为可能具有生物可及性。第一种是1.0 M的ch3cooa溶液,用CH3COOH调节pH为5,以瞄准可交换的、与碳酸盐矿物结合的金属馏分。第二种是模拟胃液的溶液(USEPA方法1340)。分析的腐蚀尺度是从美国市政当局挖掘的铅(Pb)和铜(Cu)饮用水管道中获得的,这些管道释放到水中的金属浓度预计将达到铅和铜规则(LCR)规定的阈值。虽然非晶相没有被XRD检测到,但我们的发现强调了它们对溶解金属测量浓度的潜在贡献,从化学提取的高浓度金属中推断,这些金属与衍射图中鉴定的矿物无关。因此,仅使用XRD识别结晶矿物不足以预测腐蚀水垢对流经市政配水管的饮用水造成污染的可能性,从而对人类健康构成威胁。对潜在生物可及金属组分的评价表明,对Pb、Cu和一定程度上的锌(Zn),用CH3COONa萃取得到的溶解金属浓度趋势与用US-EPA方法1340得到的结果相似,但相关性不强。总体而言,与CH3COONa方法相比,US-EPA 1340方法溶解的Pb、Cu、Mn、Fe、Cr和Sn的量明显更高,这表明,如果假设这些方法可以用于预测生物可接近金属池,则不能互换用于腐蚀尺度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Geochemistry
地学-地球化学与地球物理
CiteScore
6.10
自引率
8.80%
发文量
272
审稿时长
65 days
期刊介绍:
Applied Geochemistry is an international journal devoted to publication of original research papers, rapid research communications and selected review papers in geochemistry and urban geochemistry which have some practical application to an aspect of human endeavour, such as the preservation of the environment, health, waste disposal and the search for resources. Papers on applications of inorganic, organic and isotope geochemistry and geochemical processes are therefore welcome provided they meet the main criterion. Spatial and temporal monitoring case studies are only of interest to our international readership if they present new ideas of broad application.
Topics covered include: (1) Environmental geochemistry (including natural and anthropogenic aspects, and protection and remediation strategies); (2) Hydrogeochemistry (surface and groundwater); (3) Medical (urban) geochemistry; (4) The search for energy resources (in particular unconventional oil and gas or emerging metal resources); (5) Energy exploitation (in particular geothermal energy and CCS); (6) Upgrading of energy and mineral resources where there is a direct geochemical application; and (7) Waste disposal, including nuclear waste disposal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


