Thermodynamic analysis of nitric oxide absorption in deep eutectic solvents comprising 1,1,3,3-tetramethylguanidine
IF 2.2
3区 工程技术
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
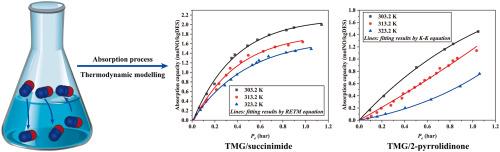
Four deep eutectic solvents (DESs) based on 1,1,3,3-tetramethylguanidine (TMG) and N-heterocycles (succinimide, 2-pyrrolidinone, 2-oxazolidone, N-methylurea) were prepared and assessed for NO capture. At 313.2 K and 1 bar, the viscosities of TMG/succinimide (TMG-Succ) and TMG/2-pyrrolidinone (TMG-Pyrr) were measured as 49.23 mPa·s and 2.85 mPa·s, respectively, while their corresponding nitric oxide (NO) uptake capacities reached 1.62 Mol·kg−1 and 1.10 Mol·kg−1 under identical conditions. Spectroscopic analysis confirmed chemisorption in TMG-Succ versus physical dissolution in TMG-Pyrr. Thermodynamic fits yielded the absorption enthalpy ΔH = −16.94 kJ·Mol−1 for TMG-Succ and − 70.1 kJ·Mol−1 for TMG-Pyrr DES. In addition, the absorption capacities of both DESs remained almost unchanged during the 10 cycles of absorption-desorption experiments, highlighting their low-viscosity, high-capacity, and energy-efficient reversible NO capture performance
含1,1,3,3-四甲基胍的深共晶溶剂中一氧化氮吸收的热力学分析
制备了4种基于1,1,3,3-四甲基胍(TMG)和n -杂环(琥珀酰亚胺、2-吡咯烷酮、2-恶唑酮、n -甲基脲)的深度共晶溶剂(DESs),并对其捕集NO的性能进行了评价。在313.2 K和1 bar条件下,TMG/琥珀酰亚胺(TMG- suc)和TMG/2-吡咯烷酮(TMG- pyrr)的黏度分别为49.23 mPa·s和2.85 mPa·s,相应的一氧化氮(NO)吸收能力分别为1.62 Mol·kg−1和1.10 Mol·kg−1。光谱分析证实了TMG-Succ的化学吸附和TMG-Pyrr的物理溶解。热力学模拟结果表明,TMG-Succ和TMG-Pyrr DES的吸附焓分别为ΔH =−16.94 kJ·Mol−1和−70.1 kJ·Mol−1。此外,在10个吸附-解吸循环实验中,两种脱氧剂的吸附能力基本保持不变,具有低粘度、高容量和节能的可逆NO捕获性能
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical Thermodynamics
工程技术-热力学
CiteScore
5.60
自引率
15.40%
发文量
199
审稿时长
79 days
期刊介绍:
The Journal of Chemical Thermodynamics exists primarily for dissemination of significant new knowledge in experimental equilibrium thermodynamics and transport properties of chemical systems. The defining attributes of The Journal are the quality and relevance of the papers published.
The Journal publishes work relating to gases, liquids, solids, polymers, mixtures, solutions and interfaces. Studies on systems with variability, such as biological or bio-based materials, gas hydrates, among others, will also be considered provided these are well characterized and reproducible where possible. Experimental methods should be described in sufficient detail to allow critical assessment of the accuracy claimed.
Authors are encouraged to provide physical or chemical interpretations of the results. Articles can contain modelling sections providing representations of data or molecular insights into the properties or transformations studied. Theoretical papers on chemical thermodynamics using molecular theory or modelling are also considered.
The Journal welcomes review articles in the field of chemical thermodynamics but prospective authors should first consult one of the Editors concerning the suitability of the proposed review.
Contributions of a routine nature or reporting on uncharacterised materials are not accepted.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


