Mechanisms of bacterial heme uptake and degradation: Diverse strategies for ring opening
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
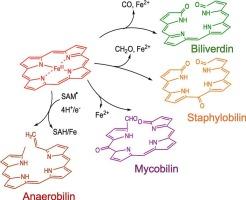
Iron acquisition by bacterial pathogens is critical for their survival and virulence within the host. Heme represents a viable source of iron by which pathogens overcome the limited iron availability and establish infection. Over the past decade several new paradigms in bacterial heme degradation have been identified. Herein, we will briefly discuss the mechanisms by which bacterial pathogens acquire and utilize heme with a particular focus on the three major classes of heme-degrading enzymes: the canonical heme oxygenases (HO), the non-canonical HOs, and the class C radical SAM methyl transferases. The canonical HO enzymes typified by the gram-negative pathogens Pseudomonas aeruginosa and Neisseriae meningitidis were shown to be structurally and mechanistically similar to the eukaryotic HO enzymes. In contrast, the non-canonical HOs of the gram-positive pathogen Staphylococcus aureus and Mycobacterium tuberculosis have a distinct ferredoxin-like structural fold and extreme heme ruffling that gives rise to alternate heme metabolites. Enteric pathogens such as E. coli O157:H7 and Vibrio cholera encode a heme-dependent radical SAM methyl transferase that opens the porphyrin ring in an oxygen-independent manner essential in the anoxic environment of the gut. All three classes of heme-degrading enzymes provide an advantage for survival within the host, while also yielding metabolites that play a role in bacterial adaptation and virulence. Therefore, a complete understanding of the distinct mechanisms of heme degradation and the role of the unique heme metabolites will provide a platform for the development of antibacterial strategies targeting heme catabolism.
细菌血红素摄取和降解的机制:不同的环打开策略
细菌病原体的铁获取对其在宿主内的生存和毒力至关重要。血红素是一种可行的铁来源,病原体通过血红素克服有限的铁可用性并建立感染。在过去的十年中,已经确定了几种细菌血红素降解的新范式。在这里,我们将简要讨论细菌病原体获取和利用血红素的机制,并特别关注三种主要的血红素降解酶:典型血红素加氧酶(HO)、非典型血红素加氧酶和C类自由基SAM甲基转移酶。以革兰氏阴性病原体铜绿假单胞菌和脑膜炎奈瑟菌为代表的典型HO酶在结构和机制上与真核HO酶相似。相反,革兰氏阳性病原体金黄色葡萄球菌和结核分枝杆菌的非典型HOs具有明显的铁氧化还原蛋白样结构褶皱和极端的血红素褶皱,从而产生交替的血红素代谢产物。大肠杆菌O157:H7和霍乱弧菌等肠道病原体编码一种血红素依赖性自由基SAM甲基转移酶,该酶以一种在肠道缺氧环境中必不可少的不依赖氧的方式打开卟啉环。这三种血红素降解酶都有利于宿主的生存,同时也产生代谢物,在细菌适应和毒力中发挥作用。因此,全面了解血红素降解的独特机制和独特血红素代谢产物的作用将为开发针对血红素分解代谢的抗菌策略提供平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


