Fe(II)-induced phase transformation of La-substituted ferrihydrite significantly influences the reduction and immobilization of Cr(VI) in soils
IF 3.6
2区 地球科学
Q1 GEOCHEMISTRY & GEOPHYSICS
引用次数: 0
Abstract
Ferrihydrite (Fh) can usually transform into phases with higher crystallinities. This may greatly alter Fh adsorption capacity for chromium ions, thereby affecting their immobilizations and fates. Foreign elements [e.g., lanthanum (La)] as impurities can become incorporated into Fh. Herein, the effect of La-substitution into Fh and the influence of the presence of Cr(VI) on Fh phase transformation and fate of Cr(VI) were investigated. X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), Electro-chemical impedance spectroscopy (EIS), and X-ray photoelectron spectroscopy (XPS) and other test methods were employed to probe phase changes after the Fe(II)-induced La-substituted Fh (La-Fh) transformations. At pH 5.5, no neoformed phase was observed until 7 d for La-Fh or pure Fh at 1 mM Fe(II) aq, but all La-Fh systems underwent transformations at 5 mM Fe(II)aq. A low pH and Fe(II)aq level favored lepidocrocite (Lp) to form, whereas goethite (Gt) was the predominant phase rather than Lp as pH increased to 7.2 with higher level Fe(II)aq. However, the La-substitution inhibits the La-Fh transformation rate and path. This made 5 % La-Fh transformed to Lp after 30 d at pH 7.2 and 1 mM Fe(II)aq. The La-release was present to some extent during the transformation process. Either the inhibition to La-Fh transformation or the La-slow release favors the retention of early-adsorbed Cr(VI) on iron-mineral surface. The added Cr(VI) might promote the La-Fh transformation even under a lower-level Fe(II), primarily attributed to the rapid reduction of Cr(VI) to Cr(III) by Fe(II), which produced more liable Fe(III) in addition to the Cr(III) that can improve the mineral conductivity, thereby enhancing the La-Fh transformation. Mechanisms responsible for the Fe(II)-induced La-Fh transformation companying the La slow-release are proposed to provide insights into the reduction and immobilization of Cr(VI) under anaerobic conditions, thus demonstrating that La-Fh is a promising material for application in Cr-contaminated site remediation.
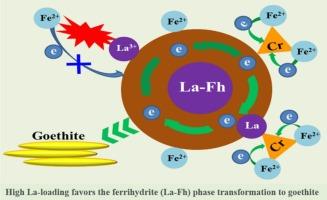
Fe(II)诱导的la -取代水合铁相变对土壤中Cr(VI)的还原和固定化有显著影响
水合铁(Fh)通常可以转变成结晶度较高的相。这可能会极大地改变Fh对铬离子的吸附能力,从而影响其固定化和命运。外来元素[如镧(La)]作为杂质可并入Fh。本文研究了la -取代成Fh的作用以及Cr(VI)的存在对Fh相变和Cr(VI)命运的影响。采用x射线衍射(XRD)、傅里叶变换红外光谱(FTIR)、电化学阻抗谱(EIS)、x射线光电子能谱(XPS)等测试方法探测Fe(II)诱导的la -取代Fh (La-Fh)转化后的相变化。在pH 5.5下,直到7天La-Fh或纯Fh在1 mM Fe(II)aq下都没有观察到新形成的相,但所有La-Fh体系在5 mM Fe(II)aq下都发生了转变。较低的pH和Fe(II)aq有利于蛭石(Lp)的形成,而当pH升高到7.2,Fe(II)aq水平升高时,针铁矿(Gt)取代Lp成为主要相。然而,la -取代抑制了La-Fh的转化速率和路径。这使得5%的La-Fh在pH 7.2和1mm Fe(II)aq条件下30天后转化为Lp。在转化过程中存在一定程度的la释放。无论是对La-Fh转化的抑制,还是对La-Fh缓释的抑制,都有利于早期吸附的Cr(VI)在铁矿物表面的保留。添加的Cr(VI)可以促进La-Fh的转化,即使在较低的Fe(II)水平下,这主要是由于Fe(II)将Cr(VI)快速还原为Cr(III),从而产生更容易的Fe(III)和Cr(III),而Cr(III)可以提高矿物的导电性,从而促进La-Fh的转化。本文提出了Fe(II)诱导的La- fh转化伴随La缓慢释放的机制,为厌氧条件下Cr(VI)的还原和固定化提供了新的见解,从而证明了La- fh是一种有前途的材料,可用于Cr污染场地的修复。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Geology
地学-地球化学与地球物理
CiteScore
7.20
自引率
10.30%
发文量
374
审稿时长
3.6 months
期刊介绍:
Chemical Geology is an international journal that publishes original research papers on isotopic and elemental geochemistry, geochronology and cosmochemistry.
The Journal focuses on chemical processes in igneous, metamorphic, and sedimentary petrology, low- and high-temperature aqueous solutions, biogeochemistry, the environment and cosmochemistry.
Papers that are field, experimentally, or computationally based are appropriate if they are of broad international interest. The Journal generally does not publish papers that are primarily of regional or local interest, or which are primarily focused on remediation and applied geochemistry.
The Journal also welcomes innovative papers dealing with significant analytical advances that are of wide interest in the community and extend significantly beyond the scope of what would be included in the methods section of a standard research paper.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


