Removal of EpCAM-positive tumor cells during intraoperative blood salvage– A pivotal multicenter clinical study (REMOVE)
IF 5.1
2区 医学
Q1 ANESTHESIOLOGY
引用次数: 0
Abstract
Background
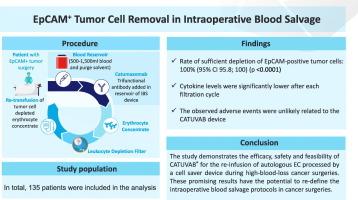
Intraoperative blood salvage can reduce the need for allogeneic blood transfusions, minimizing immunological risks and infection by reusing the patient's own blood. However, in cancer surgery, a key concern limiting its use is the potential reintroduction of viable cancer cells. Additional risks, such as infection and coagulopathy, have also contributed to its limited adoption. Irradiation of salvaged blood remains an option; however, it is time-intensive and may have detrimental effects on blood cells. These challenges highlight the need for improved technologies to enable safe application in oncological procedures. One such promising tool is CATUVAB®, which removes epithelial cell adhesion molecule (EpCAM)-positive tumor cells from salvaged blood.
Methods
In this multicenter, clinical validation study, patients undergoing major surgery for bladder cancer, gastric carcinoma, ovarian carcinoma, pancreatic carcinoma, colon/rectal carcinoma, non-small cell lung cancer, or peritoneal carcinomatosis were included. The primary objective of the study was to elucidate the efficacy of CATUVAB® in depleting intraoperatively salvaged blood of EpCAM-positive tumor cells. Secondary objectives included the determination of residual Catumaxomab antibody amount and cytokine levels in the final erythrocyte concentrate (EC).
Results
203 patients were assessed for eligibility, and 80 were included in the safety analysis, of whom 61 had EpCAM-positive tumor cells in intraoperative blood. The primary efficacy analysis demonstrated a 100 % (95 % CI (95.8 %–100 %)) rate of sufficient depletion of EpCAM-positive tumor cells (p < 0.0001). In 117 out of 131 EC samples (89 %) the catumaxomab concentration was below the limit of quantification. Additionally, cytokines IL-6 and IL-8, typically due to surgical trauma, were significantly reduced (30- to 38-fold) in ECs compared to intraoperative blood.
Conclusion
The study demonstrates the efficacy, safety and feasibility of CATUVAB® for the re-infusion of autologous EC processed by a cell salvage device during high-blood-loss cancer surgeries. These promising results have the potential to re-define the intraoperative blood salvage protocols in cancer surgeries.
术中补血过程中去除epcam阳性肿瘤细胞-一项关键的多中心临床研究(REMOVE)
背景:术中血液回收可以减少对异体输血的需求,通过重复使用患者自身血液将免疫风险和感染降至最低。然而,在癌症手术中,限制其使用的一个关键问题是有可能重新引入活的癌细胞。其他风险,如感染和凝血功能障碍,也导致其采用有限。照射回收的血液仍然是一种选择;然而,它是耗时的,可能对血细胞有有害影响。这些挑战突出表明,需要改进技术,以确保在肿瘤手术中的安全应用。CATUVAB®是一种很有前景的工具,它可以从回收的血液中去除上皮细胞粘附分子(EpCAM)阳性的肿瘤细胞。方法在这项多中心临床验证研究中,纳入了膀胱癌、胃癌、卵巢癌、胰腺癌、结肠/直肠癌、非小细胞肺癌或腹膜癌等接受大手术的患者。该研究的主要目的是阐明CATUVAB®在消耗术中存留的epcam阳性肿瘤细胞的疗效。次要目的包括最终红细胞浓缩物(EC)中残留的Catumaxomab抗体量和细胞因子水平的测定。结果203例患者入选,80例纳入安全性分析,其中61例术中血中epcam阳性肿瘤细胞。初步疗效分析显示,epcam阳性肿瘤细胞的充分耗竭率为100% (95% CI(95.8% - 100%))。0.0001)。131份EC样品中有117份(89%)的卡妥单抗浓度低于定量限。此外,细胞因子IL-6和IL-8,通常是由于手术创伤,与术中血液相比,在ECs中显著降低(30- 38倍)。结论本研究验证了CATUVAB®用于高失血癌症手术中细胞回收装置处理的自体EC再输注的有效性、安全性和可行性。这些有希望的结果有可能重新定义癌症手术中的术中血液回收方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.40
自引率
4.50%
发文量
346
审稿时长
23 days
期刊介绍:
The Journal of Clinical Anesthesia (JCA) addresses all aspects of anesthesia practice, including anesthetic administration, pharmacokinetics, preoperative and postoperative considerations, coexisting disease and other complicating factors, cost issues, and similar concerns anesthesiologists contend with daily. Exceptionally high standards of presentation and accuracy are maintained.
The core of the journal is original contributions on subjects relevant to clinical practice, and rigorously peer-reviewed. Highly respected international experts have joined together to form the Editorial Board, sharing their years of experience and clinical expertise. Specialized section editors cover the various subspecialties within the field. To keep your practical clinical skills current, the journal bridges the gap between the laboratory and the clinical practice of anesthesiology and critical care to clarify how new insights can improve daily practice.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


