Rational design, synthesis, and evaluating primary biological activities of novel HIV-1 protease inhibitors containing heteraryl acetamides as the P2 ligands
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
A series of novel potent HIV-1 protease inhibitors featuring diverse hydroxyaromatic acetanilide derivatives as P2 ligands and 4-substituted phenyl sulfonamides as P2’ ligands were designed, synthesized, and biologically evaluated. The majority of the target compounds demonstrated potent enzyme inhibitory activity with IC50 values below100 nM. Notably, compound 18h, incorporating a 2-(4-hydroxypyrimidin-5-yl) acetamide P2 ligand and a 4-methoxybenzenesulfonamide as the P2’ ligand, exhibited exceptional potency with an enzyme IC50 of 0.46 nM and antiviral EC50 of 0.26 μM against HIV-1NL4–3 strain. In addition, 18h displayed activity with EC50 value of 0.25 μM against the subtype C HIV-1 Indie strain. The extensive hydrogen-bonding interactions with the protease active site revealed in the molecular docking analysis of 18h-bound HIV-1 protease provided valuable structural insights for the rational design of next-generation HIV-1 protease inhibitors.
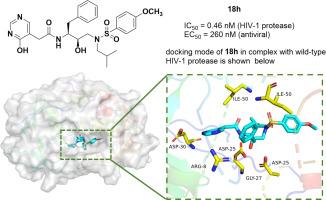
以杂芳酰乙酰胺为P2配体的新型HIV-1蛋白酶抑制剂的合理设计、合成和初步生物活性评价
设计、合成了一系列新型有效的HIV-1蛋白酶抑制剂,它们以不同的羟基乙酰苯胺衍生物为P2配体,以4-取代苯基磺酰胺为P2 '配体。大多数目标化合物表现出较强的酶抑制活性,IC50值在100 nM以下。值得注意的是,含有2-(4-羟基嘧啶-5-酰基)乙酰胺P2配体和4-甲氧基苯磺酰胺作为P2配体的化合物18h,对HIV-1NL4-3株的酶IC50为0.46 nM,抗病毒EC50为0.26 μM。此外,18h对C亚型HIV-1 Indie菌株的EC50值为0.25 μM。18h结合HIV-1蛋白酶的分子对接分析揭示了与蛋白酶活性位点广泛的氢键相互作用,为合理设计下一代HIV-1蛋白酶抑制剂提供了有价值的结构见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


