Electrochemical Conversions of Sulfinamidines into Sulfonimidoyl Fluorides
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Sulfonimidoyl fluorides, the chiral aza-isosteres of sulfonyl fluorides, have gained increasing attention as a powerful linkage agent in the sulfur(VI)-fluoride exchange reaction (SuFEx). The hexavalent sulfonimidoyl fluorides are typically prepared from organosulfur in high oxidation states. We report herein an electrochemical approach using the readily available, bench-stable, yet underexplored tetravalent sulfinamidines without external chemical oxidants. The usefulness of the obtained sulfonimidoyl fluorides is demonstrated by their versatile SuFEx reactivity.
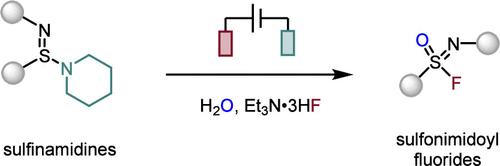
亚胺类化合物转化为磺酰酰氟化物的电化学研究。
磺酰酰氟化合物是磺酰氟化合物的手性偶氮异构体,在硫(VI)-氟交换反应(SuFEx)中作为一种强有力的连锁剂而受到越来越多的关注。六价磺胺酰氟化物通常由高氧化态的有机硫制备。我们在此报告了一种电化学方法,使用现成的,稳定的,但尚未开发的四价亚胺,不需要外部化学氧化剂。所得的磺胺酰氟化合物的用途由它们的多用途SuFEx反应性证明。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


