Cobalt-Catalyzed Alkene Alkylarylation: Access to Pyrrolidinones
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Practical cyclization and cross-coupling reactions between alkenyl-substituted alkyl bromides and arylzinc pivalates via cobalt-catalyzed sequential C(sp3)–C(sp3)/C(sp3)–C(sp2) bond formation have been disclosed, thus providing straightforward access to structurally diverse pyrrolidinones. Moreover, this approach could be also effective for the modular preparation of spirocyclic pyrrolidinones through spirocyclized alkene alkylarylation. Beyond that, rather mild conditions, synthetic simplicity, and excellent functional group compatibility show the potential applications of this method in synthetic chemistry.
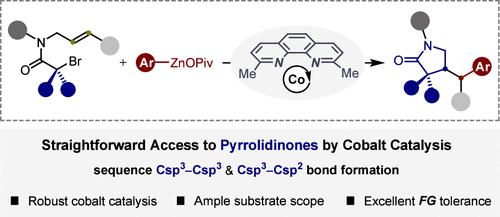
钴催化烯烃烷基化:获得吡咯烷酮。
通过钴催化的顺序C(sp3)-C(sp3)/C(sp3)-C(sp2)键形成,炔基取代的烷基溴化物和芳基戊酸锌之间的环化和交叉偶联反应已经公开,从而提供了直接获得结构多样的吡咯烷酮的途径。此外,该方法也可用于通过螺环化烯烃烷基化模块化制备螺环吡咯烷酮。除此之外,相当温和的条件、合成简单性和良好的官能团相容性显示了该方法在合成化学中的潜在应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


