Syndecan-1 regulates lipid metabolism and mitigates fibrosis during the transition from acute kidney injury to chronic kidney disease
IF 11.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Background
The transition from acute kidney injury (AKI) to chronic kidney disease (CKD) is characterized by persistent renal fibrosis, in which abnormal lipid metabolism plays a crucial role. Syndecan-1 (SDC-1) has been implicated in various tissue remodeling processes; however, its role in lipid metabolism and fibrosis during the progression from AKI to CKD is not well understood.
Methods
This study used a murine model of unilateral ischemia-reperfusion-induced AKI-to-CKD progression for in vivo analysis and employed transforming growth factor-beta (TGF-β)-induced fibrosis in Human Kidney-2 cells and primary mouse tubular epithelial cells for in vitro studies. The tubule-specific knockout and overexpression of SDC-1 mice were utilized to investigate kidney fibrosis and lipid metabolism.
Results
Following unilateral ischemia-reperfusion and TGF-β stimulation, SDC-1 expression was significantly reduced, exacerbating renal fibrosis. Notably, SDC-1 deficiency led to lipid accumulation in the kidneys, while its overexpression alleviated lipid overload and improved metabolic parameters. Furthermore, SDC-1 played a crucial role in regulating fatty acid-binding protein 7 (FABP7), and its absence resulted in increased FABP7 levels. Inhibition of FABP7 not only reduced fibrosis but also restored carnitine palmitoyltransferase 1α expression, which suggests that the SDC-1/FABP7 axis is critical for maintaining lipid homeostasis and mitigating fibrosis in the kidney.
Conclusion
These findings underscore the importance of SDC-1 in lipid metabolism and suggest that targeting lipid metabolic pathways may represent therapeutic strategies that can slow the progression of AKI to CKD.
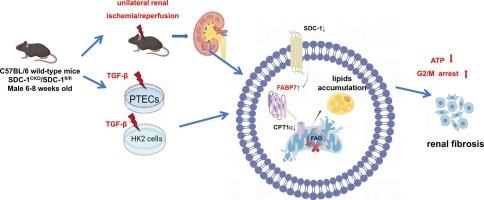
Syndecan-1调节脂质代谢,减轻急性肾损伤向慢性肾病转变过程中的纤维化。
背景:从急性肾损伤(AKI)到慢性肾脏疾病(CKD)的转变以持续的肾纤维化为特征,其中异常的脂质代谢起着至关重要的作用。Syndecan-1 (SDC-1)参与多种组织重塑过程;然而,在从AKI到CKD的过程中,其在脂质代谢和纤维化中的作用尚不清楚。方法:本研究采用小鼠单侧缺血再灌注诱导的aki向ckd进展模型进行体内分析,并采用转化生长因子β (TGF-β)诱导的人肾-2细胞和小鼠原代小管上皮细胞纤维化进行体外研究。利用小管特异性敲除和SDC-1小鼠的过表达来研究肾脏纤维化和脂质代谢。结果:单侧缺血再灌注和TGF-β刺激后,SDC-1表达明显降低,加重肾纤维化。值得注意的是,SDC-1缺乏导致肾脏脂质积累,而其过表达减轻了脂质过载并改善了代谢参数。此外,SDC-1在调节脂肪酸结合蛋白7 (FABP7)中起着至关重要的作用,其缺失导致FABP7水平升高。抑制FABP7不仅可以减少纤维化,还可以恢复肉碱棕榈酰基转移酶1α的表达,这表明SDC-1/FABP7轴对维持肾脏脂质稳态和减轻纤维化至关重要。结论:这些发现强调了SDC-1在脂质代谢中的重要性,并提示靶向脂质代谢途径可能是减缓AKI向CKD进展的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Metabolism: clinical and experimental
医学-内分泌学与代谢
CiteScore
18.90
自引率
3.10%
发文量
310
审稿时长
16 days
期刊介绍:
Metabolism upholds research excellence by disseminating high-quality original research, reviews, editorials, and commentaries covering all facets of human metabolism.
Consideration for publication in Metabolism extends to studies in humans, animal, and cellular models, with a particular emphasis on work demonstrating strong translational potential.
The journal addresses a range of topics, including:
- Energy Expenditure and Obesity
- Metabolic Syndrome, Prediabetes, and Diabetes
- Nutrition, Exercise, and the Environment
- Genetics and Genomics, Proteomics, and Metabolomics
- Carbohydrate, Lipid, and Protein Metabolism
- Endocrinology and Hypertension
- Mineral and Bone Metabolism
- Cardiovascular Diseases and Malignancies
- Inflammation in metabolism and immunometabolism
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


