Design, synthesis and anti-influenza A virus activity of N-containing heterocyclic glycyrrhetinic acid derivatives
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Glycyrrhetinic acid (GA), a bioactive triterpenoid derived from Glycyrrhiza glabra, exhibits broad-spectrum antiviral properties but suffers from poor solubility and bioavailability. To enhance its anti-influenza activity, we designed and synthesized 12 novel nitrogen-containing heterocyclic GA derivatives through structural modifications at the A-ring (C-2/C-3) and C-30 position. All compounds were evaluated against influenza A/H1N1 virus in 293T cells. At 10 μM, 10 derivatives outperformed ribavirin, with compound 12b (bearing an A-ring furazan and C-30 imidazole ester) showing the highest potency (IC50 = 2.9 μM, selectivity index SI = 38.6) representing a 7.1-fold improvement over GA (IC50 = 9.6 μM) and 3.7-fold superiority to ribavirin. Molecular docking revealed that 12b binds strongly to neuraminidase (PDB:1NN2; binding energy: −8.11 kcal/mol) via hydrogen bonds with Glu413, Asp125, and Phe100, suggesting NA as a potential target. This study demonstrates that A-ring furazan modification combined with C-30 nitrogen-containing heterocyclic incorporation significantly enhances anti-influenza activity, providing a promising lead compound (12b) for further development.
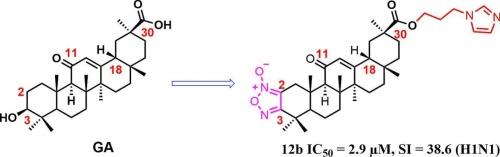
含n杂环甘草次酸衍生物的设计、合成及抗甲型流感病毒活性研究。
甘草次酸(Glycyrrhetinic acid, GA)是一种从甘草中提取的生物活性三萜,具有广谱抗病毒特性,但其溶解度和生物利用度较差。为了增强其抗流感活性,我们通过在a环(C-2/C-3)和C-30位置进行结构修饰,设计并合成了12个新的含氮杂环GA衍生物。所有化合物在293T细胞中对流感A/H1N1病毒进行了评价。10点 μM, 10衍生品优于利巴韦林,化合物12 b(轴承环furazan和正在被咪唑酯)显示最高的效力(IC50 = 2.9 μM,选择性指数SI = 38.6)代表一个7.1倍改进GA (IC50 = 9.6 μM)和3.7倍优于利巴韦林。分子对接显示12b与神经氨酸酶(PDB:1NN2;结合能:-8.11 kcal/mol)通过与Glu413、Asp125和Phe100的氢键结合,提示NA是潜在的靶标。本研究表明,a环呋喃杂环修饰与C-30含氮杂环结合可显著增强抗流感活性,为进一步开发提供了有前景的先导化合物(12b)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


