Cost-effective chiral auxiliary-assisted remote asymmetric C(sp3)–H alkylation of hydroxamic acid derivatives with glycine derivatives†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Visible-light-mediated commercially available and cost-effective chiral auxiliary-assisted radical–radical cross-coupling for the preparation of noncanonical chiral amino acids under base- and metal-free conditions has been reported. The reaction featured a broad substrate scope and excellent enantioselectivity. Besides, the products can be converted to biologically important chiral β-amino alcohols under simple procedures.
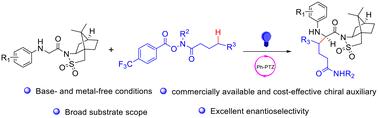
低成本手性助剂辅助羟基肟酸衍生物与甘氨酸衍生物的远端不对称C(sp3) -H烷基化反应
在无碱和无金属的条件下,用可见光介导的、商业化的、具有成本效益的手性辅助自由基-自由基交叉偶联制备了非典型手性氨基酸。该反应的底物范围广,对映体选择性好。此外,产物可以在简单的程序下转化为具有重要生物学意义的手性β-氨基醇。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


