Selective oxidation of 2,3,6-trimethylphenol into 2,3,5-trimethyl-1,4-benzoquinone with dioxygen over heterogeneous Co catalysts†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The selective oxidation of 2,3,6-trimethylphenol is an important approach to prepare 2,3,5-trimethyl-1,4-benzoquinone, which is a key intermediate for the synthesis of vitamin E. Previous studies have shown that Co–N–C materials prepared through a pyrolysis process at temperatures between 250 and 400 °C exhibit high catalytic activity for the oxidation of 2,3,6-trimethylphenol, rather than the typical range of 600 to 1000 °C. In addition to these unusual findings, activated carbon-supported Co(Phen)2 after pyrolysis showed significantly lower catalytic activity compared to its unsupported counterpart, and the pyrolyzed Co(Salen)2 complex did not display the same high catalytic activity as the homogeneous counterpart. The presence of pyridinic nitrogen (N) on the surface, rather than pyrrolic N, is closely associated with the efficient formation of 2,3,5-trimethyl-1,4-benzoquinone. Active catalytic sites with highly disordered structures are formed alongside the first apparent weight loss during pyrolysis. These results provide important insights into the oxidation of phenolic compounds and catalyst design.
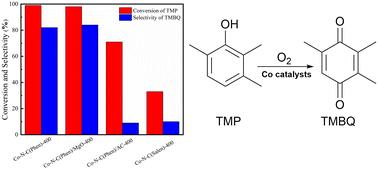
非均相Co催化剂上2,3,6-三甲基苯酚选择性氧化制备2,3,5-三甲基-1,4-苯醌
2,3,6-三甲基苯酚的选择性氧化是制备2,3,5-三甲基-1,4-苯醌的重要途径,而2,3,5-三甲基-1,4-苯醌是合成维生素e的关键中间体。先前的研究表明,通过热解工艺制备的Co-N-C材料在250 ~ 400℃的温度范围内对2,3,6-三甲基苯酚的氧化表现出较高的催化活性,而不是典型的600 ~ 1000℃范围。除了这些不寻常的发现外,活性炭负载Co(Phen)2在热解后的催化活性明显低于未负载Co(Phen)2,并且热解后的Co(Salen)2配合物没有表现出与均相配合物相同的高催化活性。表面存在吡啶氮(N)而非吡啶氮,与2,3,5-三甲基-1,4-苯醌的有效生成密切相关。具有高度无序结构的活性催化位点在热解过程中伴随着第一次明显的重量损失而形成。这些结果为酚类化合物的氧化和催化剂设计提供了重要的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


