DFT investigation of mechanism, regioselectivity, and chemoselectivity in rhodium(iii)-catalyzed oxidative cyclization of chalcones with internal alkynes†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Density functional theory (DFT) was used to examine the mechanism, regioselectivity, and chemoselectivity in the rhodium(iii)-catalyzed oxidative cyclization of chalcones with internal alkynes. The computational findings indicate that the entire reaction comprises two fundamental catalytic cycles. Initially, the reaction undergoes the C–H activation, followed by migratory insertion, reductive elimination, and hydrolysis to form an enol, thereby completing the first catalytic cycle. Subsequently, the enol undergoes O–H deprotonation, migratory insertion, reductive elimination, hydrolysis, and retro-aldol reaction to generate the final product. In the case of asymmetric alkynes, distortion–interaction analysis indicates that the regioselectivity is influenced by steric effects during the alkyne insertion step, while the chemoselectivity for the Rh–C or Rh–O bonds is predominantly governed by spatial hindrance and electronic influence of the substrate. A linear fitting of charges in NPA (natural population analysis) for various substituents on different chalcones reveals that the charge of the starting material plays an important role in its ability to undergo a successful (4 + 1) cyclization. This study contributes to a deeper understanding of the fundamental reaction mechanisms and offers significant insights for the rational design of related catalytic processes.
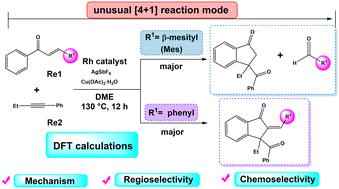
铑(iii)催化查尔酮内炔氧化环化反应机理、区域选择性和化学选择性的DFT研究
采用密度泛函理论(DFT)研究了铑(III)催化查尔酮与内炔氧化环化反应的机理、区域选择性和化学选择性。计算结果表明,整个反应包括两个基本的催化循环。最初,反应经过C-H活化,然后迁移插入,还原消除,水解形成烯醇,从而完成第一个催化循环。随后,烯醇经过O-H去质子化、迁移插入、还原消除、水解和反醛醇反应生成最终产物。在不对称炔的情况下,扭曲相互作用分析表明,区域选择性受到炔插入步骤中空间效应的影响,而Rh-C或Rh-O键的化学选择性主要受空间位阻和底物电子影响的影响。在NPA(自然种群分析)中对不同查尔酮上不同取代基的电荷进行了线性拟合,揭示了起始材料的电荷对其成功进行(4 + 1)环化的能力起着重要作用。该研究有助于加深对基本反应机理的认识,并为相关催化过程的合理设计提供重要见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


