Photocatalytic decarboxylative deuteration of lauric acid with heavy water for sustainable synthesis of deuterated alkanes†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
As a simple model system for sustainable synthesis of deuterated alkanes, photocatalytic decarboxylative deuteration of lauric acid (dodecanoic acid) was explored employing heavy water (2H2O, D2O) as the deuterium source and titanium dioxide (TiO2) photocatalysts loaded with metal cocatalysts (Au, Pt or Pd), without requiring any other reagents. The photocatalysts effectively facilitated the production of monodeuterated undecane ([2H1]undecane, C11H23D). The alkyl radical degenerated from lauric acid through decarboxylation couples with the deuterium radical generated from heavy water, resulting in the formation of the deuterated undecane. Among the tested photocatalysts, a gold-loaded TiO2 (Au/TiO2) photocatalyst pre-dried before use achieved a 15.3% yield of deuterated undecane after 3 hours of photocatalytic reaction, with the deuteration ratio (Rd) in the obtained undecane reaching 85.1%. While extending the reaction time increased the overall yield, it led to a lower Rd. The Rd did not reach 100% since the alkyl radical intermediate also reacts with lauric acid or another alkyl radical to form non-deuterated undecane (C11H24) or docosane (C22H46), respectively as byproducts. The reaction mechanism of this photocatalytic system was elucidated using ESR measurement with radical trapping. This study offers a model methodology for the efficient synthesis of deuterated compounds, with potential applications in various fields such as pharmaceuticals.
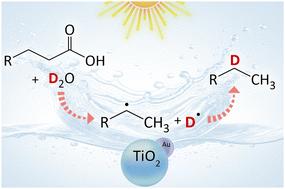
重水光催化月桂酸脱羧氘化合成氘化烷烃
作为一种简单的可持续合成氘化烷烃的模型体系,以重水(2H2O, D2O)为氘源,二氧化钛(TiO2)光催化剂负载金属助催化剂(Au, Pt或Pd),探索光催化脱羧氘化月桂酸(十二烷酸),无需其他试剂。光催化剂有效促进了单氘化十一烷([2H1]十一烷,C11H23D)的生成。月桂酸脱羧后的烷基自由基与重水生成的氘自由基偶联,形成氘化十一烷。在所测试的光催化剂中,一种负载金的TiO2 (Au/TiO2)光催化剂在使用前进行预干燥,经过3小时的光催化反应,氘化十一烷的产率达到15.3%,所得十一烷的氘化比(Rd)达到85.1%。反应时间的延长提高了总产率,但降低了Rd。Rd没有达到100%,因为烷基自由基中间体还与月桂酸或另一烷基自由基反应,分别生成非氘化十一烷(C11H24)或十二烷(C22H46)作为副产物。用ESR法分析了该光催化体系的反应机理。本研究为氘化化合物的高效合成提供了一种模型方法,在制药等各个领域具有潜在的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


