Ag2O-modified MnO2 enhanced electrocatalytic propylene epoxidation with water as the sole oxygen source†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Propylene oxide, as a pivotal chemical raw material with an annual demand exceeding 20 million tons, faces challenges in traditional production processes such as high energy consumption, large carbon emissions, and severe environmental pollution. Electrocatalytic propylene epoxidation, utilizing renewable electricity instead of chemical oxidants and water as a clean oxygen source, is a green and safe synthesis strategy for propylene oxide. However, it is still necessary to solve the problem of low yield. In this work, we prepared a 0.5Ag2O/MnO2 catalyst and obtained a high yield of 13.4 g m−2 h−1, which displayed a 20-fold improvement compared to MnO2 (0.6 g m−2 h−1). This was attributed to the abundant oxygen vacancies and active sites, which would weaken the interaction of Mn–O bonds and promote the activation of propylene.
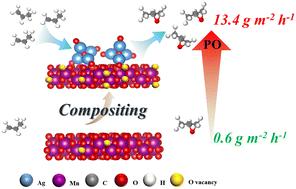
ag20改性MnO2增强水为唯一氧源的电催化丙烯环氧化反应
环氧丙烷作为年需求量超过2000万吨的关键化工原料,其传统生产工艺面临着能耗高、碳排放大、环境污染严重等挑战。电催化丙烯环氧化是一种绿色、安全的环氧丙烷合成策略,利用可再生电力代替化学氧化剂和水作为清洁的氧源。但是,仍然需要解决产量低的问题。在这项工作中,我们制备了0.5Ag2O/MnO2催化剂,获得了13.4 g m−2 h−1的高产率,与MnO2 (0.6 g m−2 h−1)相比,提高了20倍。这是由于丰富的氧空位和活性位点削弱了Mn-O键的相互作用,促进了丙烯的活化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


