Two opposing redox signals mediated by 2-cys peroxiredoxin shape the redox proteome during photosynthetic induction
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Photosynthetic induction, characterized by the lag in CO2 assimilation rates during transition from darkness to light, has traditionally been attributed to Rubisco activase activity and stomatal opening. Yet, the faster induction of photosynthesis in the 2-Cys peroxiredoxins (Prxs) mutant (2cpab) suggested a role for oxidative signals in regulating photosynthetic rates, although the underlying molecular mechanism remains unclear. SPEAR, a redox proteomics approach, was used to systematically map redox changes occurring during photosynthesis induction and to unravel the role of 2-Cys Prxs in shaping these redox alterations. No significant difference was observed in protein expression levels between WT and 2cpab plants, suggesting that protein abundance does not account for the 2cpab phenotype. During the transition from dark to low light, 82 and 54 cysteine-containing peptides were reduced or oxidized, respectively, in WT plants. Most redox-regulated cysteines in photosynthetic proteins were found oxidized in the dark and became reduced in response to light. A reverse pattern was observed among redox-regulated cysteines in proteins involved in starch degradation and chloroplast glycolysis, which shifted from a reduced to an oxidized state in response to light. These findings demonstrate the initiation of two opposing redox responses, affecting distinct sets of metabolic proteins during the induction phase. Remarkably, a significantly lower number of cysteines were reduced or oxidized in 2cpab plants, highlighting the crucial role 2-Cys Prxs play in shaping both signals. Taken together, rotational shifts between metabolic pathways during the photosynthesis induction phase are regulated by two opposing redox signals mediated by 2-Cys Prx activity.
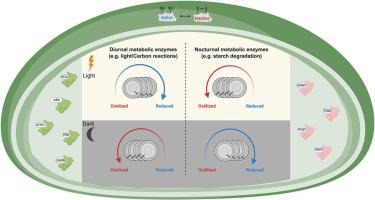
在光合诱导过程中,由2-cys过氧化物还氧蛋白介导的两个相反的氧化还原信号形成氧化还原蛋白质组
光合作用诱导的特征是在从黑暗到光明的过渡过程中CO2同化速率的滞后,传统上认为是Rubisco激活酶活性和气孔开放。然而,在2-Cys过氧化物还毒素(Prxs)突变体(2pab)中,更快的光合作用诱导表明氧化信号在调节光合速率中起作用,尽管潜在的分子机制尚不清楚。SPEAR是一种氧化还原蛋白质组学方法,用于系统地绘制光合作用诱导过程中发生的氧化还原变化,并揭示2-Cys Prxs在形成这些氧化还原变化中的作用。在WT和2cpab植物之间没有观察到蛋白表达水平的显著差异,这表明蛋白丰度不能解释2cpab表型。在从暗光到弱光的过渡过程中,WT植物分别有82和54种含半胱氨酸的肽被还原或氧化。光合蛋白中大多数氧化还原调节的半胱氨酸在黑暗中被氧化,并在光照下被还原。在涉及淀粉降解和叶绿体糖酵解的蛋白质中,氧化还原调节的半胱氨酸中观察到相反的模式,它们在响应光的情况下从还原状态转变为氧化状态。这些发现证明了两种相反的氧化还原反应的启动,在诱导阶段影响不同的代谢蛋白组。值得注意的是,在2pab植物中,半胱氨酸被还原或氧化的数量明显减少,这突出了2-Cys Prxs在形成这两种信号中所起的关键作用。综上所述,光合作用诱导阶段代谢途径之间的旋转变化是由2-Cys Prx活性介导的两个相反的氧化还原信号调节的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


