Accurate antibiotic accumulation in Enterobacteriaceae isolates expressing efflux pumps
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
In Enterobacteriaceae, antibiotic susceptibility is frequently influenced by mechanisms such as membrane modifications, target site mutations, and enzymatic resistance barriers. Recently, there has been a notable rise in Klebsiella pneumoniae, Escherichia coli, and Enterobacter cloacae isolates exhibiting antibiotic resistance in hospital settings. Of particular concern, some resistant isolates employ membrane-associated resistance mechanisms that significantly lower intracellular antibiotic concentrations, reducing them below the threshold required for therapeutic efficacy. Advancements in methods for quantifying drug accumulation within bacterial cells have provided critical insights into these resistance mechanisms. A key step in these studies relies on cell lysis to release intracellular contents including antibacterial molecules for precise quantification. However, current lysis methods are often time-consuming, underscoring the need for a robust, efficient approach to accurately measure intracellular antibiotic concentrations in isolates exhibiting various levels of efflux pump activity. In this study, we developed a rapid and reliable lysis protocol that minimizes the risk of drug alteration while enabling precise and reproducible measurement of intracellular antibiotic concentrations allowing an evidence-based study of efflux in resistant clinical strains of Enterobacteriaceae. This approach holds significant promise for enhancing our understanding of membrane-associated resistance mechanisms and for informing the optimization of treatment strategies.
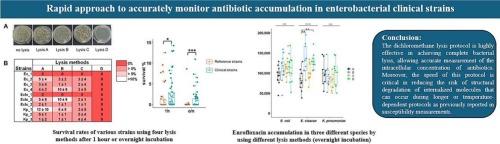
表达外排泵的肠杆菌科分离物中抗生素的准确积累
在肠杆菌科中,抗生素敏感性经常受到膜修饰、靶点突变和酶抗性屏障等机制的影响。最近,在医院环境中肺炎克雷伯菌、大肠杆菌和阴沟肠杆菌分离株表现出抗生素耐药性的病例显著增加。特别值得关注的是,一些耐药菌株采用膜相关耐药机制,显著降低细胞内抗生素浓度,使其低于治疗效果所需的阈值。定量细菌细胞内药物积累方法的进步为这些耐药机制提供了重要的见解。这些研究的关键步骤依赖于细胞裂解释放细胞内内容物,包括抗菌分子,以进行精确定量。然而,目前的裂解方法往往是耗时的,强调需要一个强大的,有效的方法来准确测量细胞内抗生素浓度的分离表现出不同水平的外排泵活性。在这项研究中,我们开发了一种快速可靠的裂解方案,最大限度地降低了药物改变的风险,同时能够精确和可重复地测量细胞内抗生素浓度,从而对肠杆菌科耐药临床菌株的外排进行循证研究。这种方法对增强我们对膜相关耐药机制的理解和优化治疗策略具有重要的意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochimica et biophysica acta. Biomembranes
生物-生化与分子生物学
CiteScore
8.20
自引率
5.90%
发文量
175
审稿时长
2.3 months
期刊介绍:
BBA Biomembranes has its main focus on membrane structure, function and biomolecular organization, membrane proteins, receptors, channels and anchors, fluidity and composition, model membranes and liposomes, membrane surface studies and ligand interactions, transport studies, and membrane dynamics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


