Hyperosmolarity-induced activation of PIEZO1 engages detrimental calcium/oxidative stress signaling and adaptive catalase response in renal inner medullary collecting duct (mIMCD3) cells
IF 3.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular cell research
Pub Date : 2025-08-07
DOI:10.1016/j.bbamcr.2025.120041
引用次数: 0
Abstract
The collecting duct (CD) is the final segment of the renal nephron and is involved in the fine regulation of osmotic and ionic homeostasis. Its medullary segment is continuously exposed to a wide spectrum of osmotic gradients and resultant osmotic stress. Strikingly, the expression of the mechanically activated non-selective cationic and Ca2+-permeable transduction ion channel PIEZO1 is most prominent in inner medullary CD (IMCD) cells, yet its functions there are still not well understood. We hypothesized increased PIEZO1 expression in the IMCD could be linked to its hyperosmotic stress environment. Using the mouse mIMCD3 cell line, which has been used to characterize hyperosmotic stress-induced cell death, we demonstrate twice as much PIEZO1 expression compared to proximal tubule (WKPT-0293 Cl.2) or cortical CD (mCCD(cl.1)) cell lines. Hyperosmolarity/−tonicity by addition of NaCl ± urea to the culture medium (+ 100–300 mosmol/l) or PIEZO1 agonist Yoda1 (20 μmol/l) decreased mIMCD3 cell viability assayed by MTT, which were antagonized by PIEZO1 inhibitors GsMTx4 (2.5 μmol/l) and salvianolic acid (SalB, 10 μmol/l). PIEZO1 activation by hyperosmolarity and agonists (Yoda1, Jedi1) increased Ca2+ influx, downstream reactive oxygen species (ROS), in particular mitochondrial superoxide (O2•-) formation, and subsequent adaptive ROS-decomposing catalase expression and activity that were sensitive to PIEZO1 antagonists (GsMTx4, SalB). Hence, the data demonstrate hyperosmolarity/−tonicity of the kidney elicits PIEZO1 activation, mitochondrial ROS formation and cell death that are partially countered by catalase-mediated stress adaptation.
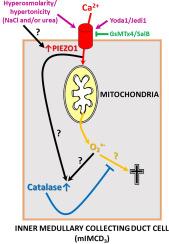
高渗透压诱导的PIEZO1激活在肾髓内收集管(mIMCD3)细胞中参与有害的钙/氧化应激信号和适应性过氧化氢酶反应。
集合管是肾元的最后一段,参与渗透和离子稳态的精细调节。它的髓质段持续暴露于广泛的渗透梯度和由此产生的渗透应力。引人注目的是,机械激活的非选择性阳离子和Ca2+渗透性转导离子通道PIEZO1的表达在髓内CD (IMCD)细胞中最为突出,但其功能仍未得到很好的理解。我们假设在IMCD中增加的PIEZO1表达可能与其高渗应激环境有关。使用小鼠mIMCD3细胞系(已用于表征高渗应激诱导的细胞死亡),我们证明PIEZO1的表达量是近端小管(WKPT-0293 Cl.2)或皮质CD(mCCD(cl.1))细胞系的两倍。采用MTT法测定,在培养基(+100-300 μmol/l)或PIEZO1激动剂Yoda1(20 μmol/l)中加入NaCl±尿素的高渗透性/-强直性可降低mIMCD3细胞活力,而PIEZO1抑制剂GsMTx4(2.5 μmol/l)和丹酚酸(SalB, 10 μmol/l)可拮抗细胞活力。高渗透压和激动剂(Yoda1, Jedi1)激活PIEZO1会增加Ca2+内流,下游活性氧(ROS),特别是线粒体超氧化物(O2•-)的形成,以及随后对PIEZO1拮抗剂(GsMTx4, SalB)敏感的适应性ROS分解过氧化氢酶的表达和活性。因此,数据表明,肾脏的高渗透压/-强直性引发PIEZO1激活,线粒体ROS形成和细胞死亡,部分被过氧化氢酶介导的应激适应所抵消。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.00
自引率
2.00%
发文量
151
审稿时长
44 days
期刊介绍:
BBA Molecular Cell Research focuses on understanding the mechanisms of cellular processes at the molecular level. These include aspects of cellular signaling, signal transduction, cell cycle, apoptosis, intracellular trafficking, secretory and endocytic pathways, biogenesis of cell organelles, cytoskeletal structures, cellular interactions, cell/tissue differentiation and cellular enzymology. Also included are studies at the interface between Cell Biology and Biophysics which apply for example novel imaging methods for characterizing cellular processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


