Defective collagen VI-NG2 axis impairs pericyte balance between proliferation and quiescence in COLVI-related myopathies
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-08-06
DOI:10.1016/j.bbadis.2025.168012
引用次数: 0
Abstract
Collagen VI-related myopathies (COLVI-RMs) are rare genetic disorders caused by impaired assembly and secretion of COLVI, a key extracellular matrix (ECM) protein. COLVI deficiency alters ECM architecture and biomechanics, leading to progressive muscle fiber damage and connective tissue abnormalities. While pericytes are emerging as key players in muscle regeneration due to their myogenic potential, their role in COLVI-RMs remains unclear.
This study investigates pericyte involvement in COLVI-RMs, focusing on the interaction between COLVI and neural/glial antigen 2 (NG2), a proteoglycan expressed on pericyte membranes.
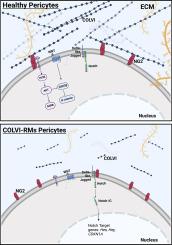
Muscle biopsies from COLVI-RMs patients revealed abnormal pericyte distribution, reduced vessel coverage, and thickened capillary basement membranes. In vitro, healthy pericytes formed a dense COLVI network, while COLVI-RM-derived pericytes displayed a disrupted matrix and impaired cell-ECM interaction.
Proximity ligation assays demonstrated a significant reduction in COLVI-NG2 binding in COLVI-RM pericytes, correlating with altered balance between proliferative and quiescent states. In turn, defects in signaling pathways related to proliferation (Akt/mTOR and Wnt/β-catenin pathways) and quiescence (N-cadherin, Notch3, FOXO3A) were identified, revealing a marked quiescent state. In vitro inhibition of the COLVI-NG2 binding in healthy pericytes reproduced these pathological features, underscoring the functional relevance of this molecular axis.
Taken together, the data here reported revealed an unexpected role of NG2-COLVI binding on pericytes status. It follows that the impairment of functional binding between NG2 and COLVI could have important consequences on the pericytes myogenic potential in COLVI-RMs, and consequently on the muscle regeneration. Finally, targeting defective pericytes could provide potential therapeutic strategies for these debilitating diseases.
胶原VI-NG2轴缺陷损害colvi相关肌病中周细胞增殖和静止之间的平衡。
胶原vi相关肌病(COLVI- rms)是一种罕见的遗传性疾病,由关键的细胞外基质(ECM)蛋白COLVI的组装和分泌受损引起。COLVI缺乏改变ECM结构和生物力学,导致进行性肌纤维损伤和结缔组织异常。虽然周细胞由于其成肌潜能而成为肌肉再生的关键角色,但它们在COLVI-RMs中的作用尚不清楚。本研究探讨了COLVI- rms与周细胞的关系,重点研究了COLVI与神经/胶质抗原2 (NG2)的相互作用,NG2是一种在周细胞膜上表达的蛋白多糖。COLVI-RMs患者的肌肉活检显示周细胞分布异常,血管覆盖减少,毛细血管基底膜增厚。在体外,健康的周细胞形成密集的COLVI网络,而COLVI- rm衍生的周细胞显示基质破坏和细胞- ecm相互作用受损。近距离连接实验表明COLVI-RM周细胞中COLVI-NG2结合显著减少,这与增殖和静止状态之间平衡的改变有关。进而发现与增殖相关的信号通路(Akt/mTOR和Wnt/β-catenin通路)和静止相关的信号通路(N-cadherin、Notch3、FOXO3A)存在缺陷,显示出明显的静止状态。在健康周细胞中COLVI-NG2结合的体外抑制再现了这些病理特征,强调了该分子轴的功能相关性。综上所述,本文报告的数据揭示了NG2-COLVI结合对周细胞状态的意想不到的作用。由此可见,NG2与COLVI之间功能结合的损害可能对COLVI- rms的周细胞成肌电位产生重要影响,从而影响肌肉再生。最后,靶向缺陷周细胞可能为这些衰弱性疾病提供潜在的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


