Synthesis and crystal structure of a new isomer of poly[di-μ3-cyanido-μ-2,6-dimethylpyrazine-dicopper(I)]
IF 0.6
Q4 CRYSTALLOGRAPHY
Acta Crystallographica Section E: Crystallographic Communications
Pub Date : 2025-08-01
DOI:10.1107/S2056989025006267
引用次数: 0
Abstract
In the crystal structure of the title compound, the copper(I) cations are linked by the cyanide anions into layers that are additionally connected by the 2,6-dimethylpyrazine ligands into a 3D network. The title compound represents a new isomer of Cu2(CN)2(2,6-dimethylpyrazine), which has already been reported in the literature.
The title compound, [Cu2(CN)2(C6H8N2)]n or Cu2(CN)2(2,6-dimethylpyrazine), was prepared by the reaction of copper(I) cyanide with 2,6-dimethylpyrazine in water. Its asymmetric unit consists of two crystallographically independent copper(I) cations and cyanide anions as well as one crystallographically independent 2,6-dimethylpyrazine ligand in general positions. Each copper cation is fourfold coordinated by one N atom of the 2,6-dimethylpyrazine ligand and three cyanide anions, that are disordered so that each C and N position has mixed occupancy with a ratio between N and C of 94:6 and 77:23. The copper cations are linked by the cyanide atoms into layers that are further connected into a 3D network by the 2,6-dimethylpyrazine ligands. Powder X-ray diffraction (PXRD) proves that a pure crystalline phase has been obtained. It is noted that this crystal structure represents a new isomer of Cu2(CN)2(2,6-dimethylpyrazine), which has already been reported in the literature [Chesnut et al. (2001#). J. Chem. Soc. Dalton Trans. pp. 2567–2580].
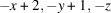
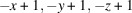
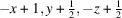
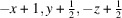
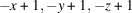
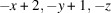
聚[二μ3-氰-μ-2,6-二甲基吡嗪-二铜]新异构体的合成与晶体结构
用氰化铜(I)与2,6-二甲基吡嗪在水中反应制备了标题化合物[Cu2(CN)2(C6H8N2)] n或Cu2(CN)2(2,6-二甲基吡嗪)。它的不对称单元由两个晶体独立的铜(I)阳离子和氰化物阴离子以及一个晶体独立的2,6-二甲基吡嗪配体在一般位置组成。每个铜阳离子是由2,6-二甲基吡嗪配体中的一个N原子和三个氰化物阴离子四倍配位的,它们是无序的,因此每个C和N位置都是混合占据的,N和C的比例为94:6和77:23。铜阳离子由氰化物原子连接成层,再由2,6-二甲基吡嗪配体连接成三维网络。粉末x射线衍射(PXRD)证实获得了纯晶相。值得注意的是,这种晶体结构代表了Cu2(CN)2(2,6-二甲基吡嗪)的一种新的异构体,这在文献中已经有报道[Chesnut et al.(2001▸)]。j .化学。Soc。道尔顿反式。页2567 - 2580)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Crystallographica Section E: Crystallographic Communications
Chemistry-Chemistry (all)
CiteScore
1.90
自引率
0.00%
发文量
351
审稿时长
3 weeks
期刊介绍:
Acta Crystallographica Section E: Crystallographic Communications is the IUCr''s open-access structural communications journal. It provides a fast, simple and easily accessible publication mechanism for crystal structure determinations of inorganic, metal-organic and organic compounds. The electronic submission, validation, refereeing and publication facilities of the journal ensure rapid and high-quality publication of fully validated structures. The primary article category is Research Communications; these are peer-reviewed articles describing one or more structure determinations with appropriate discussion of the science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


