Isotypism and phase transitions of (NH4)M(HSO4)(SO4)(H2O)2 (M = Fe, Co and Ni) compounds
IF 0.6
Q4 CRYSTALLOGRAPHY
Acta Crystallographica Section E: Crystallographic Communications
Pub Date : 2025-08-01
DOI:10.1107/S2056989025006395
引用次数: 0
Abstract
The (NH4)M(HSO4)(SO4)(H2O)2 (M = Fe, Co, Ni) compounds are isotypic and exhibit a phase transition from an ordered crystal structure at T = 100 K to a disordered crystal structure at T = 296 K.
A systematic crystallization study of {(NH4)[M(HSO4)(SO4)(H2O)2]}n compounds, catena-poly[ammonium [[diaquametal(II)]-μ-(hydrogen sulfato)-μ-sulfato]], revealed that crystals of the M = Fe, Co and Ni members could be grown by evaporation of mixed equimolar aqueous solutions of NH4HSO4 and the corresponding MSO4 sulfate, whereas for M = Mn, Cu, Zn different products were obtained under these conditions. The (NH4)M(HSO4)(SO4)(H2O)2 compounds (M = Fe, Co, Ni) show a similar behavior to the magnesium analogue, i.e. a reversible structural phase transition from an ordered triclinic crystal structure at T = 100 K (Z = 2) to a disordered triclinic structure at T = 296 K (Z = 1). The symmetry relationship between the structure at 296 K and the superstructure at 100 K is of the isomorphic type with index 2. At 100 K, the [MO4(OH2)2] octahedra are linked by distinct [SO3(OH)] and [SO4] tetrahedra into chains. Adjacent chains are linked by very strong hydrogen bonds (O⋯O ≃ 2.5 Å) between the two types of sulfate tetrahedra into layers. These layers are held together by hydrogen-bonding interactions of medium-to-weak strength between the ammonium cations and water molecules. At 296 K, the H atoms of the ammonium tetrahedron and the H atom between two symmetry-related sulfate groups are disordered. Quantitative structural comparisons are made between the isotypic (NH4)M(HSO4)(SO4)(H2O)2 structures (M = Mg, Fe, Co, Ni) at 296 K and 100 K, respectively.
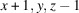
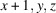
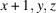
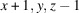
(NH4)M(HSO4)(SO4)(H2O)2 (M = Fe, Co和Ni)化合物的同型性和相变
对{(NH4)[M(HSO4)(SO4)(H2O)2]} n化合物链-聚[铵[[双水金属(II)]-μ-(硫化氢)-μ-硫-脂肪]]的结晶过程进行了系统的研究,发现在NH4HSO4和相应的MSO4硫酸盐混合等摩尔水溶液中蒸发可以生长出M = Fe, Co和Ni成员的晶体,而M = Mn, Cu, Zn在这些条件下可以得到不同的产物。(NH4)M(HSO4)(SO4)(H2O)2化合物(M = Fe, Co, Ni)表现出与镁类似物相似的行为,即从T = 100 K (Z = 2)的有序三斜晶体结构到T = 296 K (Z = 1)的无序三斜结构的可逆结构相变。296 K处结构与100 K处上部结构的对称关系为指数为2的同构型。在100k时,[MO4(OH2)2]八面体由不同的[SO3(OH)]和[SO4]四面体连接成链。相邻的链由两种类型的硫酸盐四面体之间的非常强的氢键(O⋯O≃2.5 Å)连接成层。这些层是由氢键的相互作用,在中弱强度的铵离子和水分子之间保持在一起。在296 K时,铵四面体的H原子和两个对称相关的硫酸盐基团之间的H原子无序。分别在296 K和100 K下对同型(NH4)M(HSO4)(SO4)(H2O)2 (M = Mg, Fe, Co, Ni)结构进行了定量结构比较。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Crystallographica Section E: Crystallographic Communications
Chemistry-Chemistry (all)
CiteScore
1.90
自引率
0.00%
发文量
351
审稿时长
3 weeks
期刊介绍:
Acta Crystallographica Section E: Crystallographic Communications is the IUCr''s open-access structural communications journal. It provides a fast, simple and easily accessible publication mechanism for crystal structure determinations of inorganic, metal-organic and organic compounds. The electronic submission, validation, refereeing and publication facilities of the journal ensure rapid and high-quality publication of fully validated structures. The primary article category is Research Communications; these are peer-reviewed articles describing one or more structure determinations with appropriate discussion of the science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


