The gut microbiota in cancer immunity and immunotherapy
IF 19.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
The human gastrointestinal tract harbors trillions of microorganisms, including bacteria, fungi, and viruses, to form the gut microbiota. Cumulative evidence has demonstrated the critical impact of gut microbes on cancer immunity. In cancer, an altered gut microbiota enriched with pathogenic bacteria can actively promote immune evasion and disrupt antitumor immunity, thereby supporting tumor growth and survival. Conversely, beneficial commensal bacteria (e.g., Lactobacillus and Bifidobacterium) have emerged as therapeutic probiotics for cancer prevention and as adjuvants for cancer therapy. The gut microbiota is also closely linked to the efficacy of immunotherapy. This review summarizes the effects of pathogenic bacteria and beneficial commensals, including T cells, B cells, natural killer cells, innate lymphoid cells, and myeloid-derived suppress cells, on various innate and adaptive immune cell populations in cancer. It also explores the mechanisms by which the gut microbiota influences immunotherapy efficacy, such as the modulation of innate immune cells and CD8+ T cells. Given its importance, an increasing number of studies have developed approaches to target the gut microbiota to improve immunotherapy outcomes and reduce immune-related adverse events. These strategies include antimicrobial intervention, probiotics, prebiotics/dietary modifications, microbial metabolites, phage therapy, and fecal microbiota transplantation. This review also evaluates clinical applications that use the gut microbiota to predict immunotherapy outcomes. Overall, the current understanding of host‒microbe interactions within the tumor microenvironment has laid a critical foundation for the translation of microbiota research into clinical practice, ultimately benefiting patients.
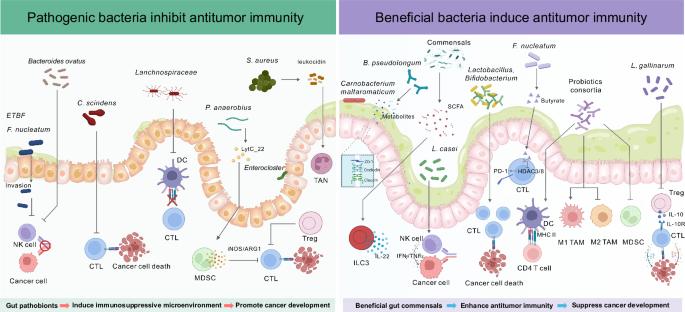
肠道微生物群在癌症免疫和免疫治疗中的作用。
人类的胃肠道孕育着数以万亿计的微生物,包括细菌、真菌和病毒,形成肠道微生物群。越来越多的证据表明,肠道微生物对癌症免疫有重要影响。在癌症中,富含致病菌的改变的肠道微生物群可以积极促进免疫逃避和破坏抗肿瘤免疫,从而支持肿瘤的生长和存活。相反,有益的共生菌(如乳酸菌和双歧杆菌)已经成为预防癌症的治疗性益生菌和癌症治疗的佐剂。肠道微生物群也与免疫疗法的疗效密切相关。本文综述了致病菌和有益共生菌,包括T细胞、B细胞、自然杀伤细胞、先天淋巴样细胞和髓源性抑制细胞,对肿瘤中各种先天和适应性免疫细胞群的影响。它还探讨了肠道微生物群影响免疫治疗效果的机制,如先天免疫细胞和CD8+ T细胞的调节。鉴于其重要性,越来越多的研究已经开发出针对肠道微生物群的方法,以改善免疫治疗结果并减少免疫相关的不良事件。这些策略包括抗菌素干预、益生菌、益生元/饮食调整、微生物代谢物、噬菌体治疗和粪便微生物群移植。本综述还评估了使用肠道菌群预测免疫治疗结果的临床应用。总的来说,目前对肿瘤微环境中宿主-微生物相互作用的理解为微生物群研究转化为临床实践奠定了关键基础,最终使患者受益。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


