One-pot Mo(CO)6-facilitated transformation of anilines into benzophenones via in situ pyridinium reconfiguration
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A novel, mechanochemical, one-pot transformation of anilines to benzophenones using molybdenum hexacarbonyl (Mo(CO)6) was achieved, avoiding pre-functionalization steps inherent in such conversions. The reaction capitalizes on the activation of the C(sp2)–NH2 bond in anilines via in situ formation of pyridinium salts: a strategy impeded by the inertness of the C–N bond. For this envisioned methodology, pyrylium tetrafluoroborate was used to convert anilines into reactive pyridinium intermediates, which undergo carbonylation in the presence of Mo(CO)6. The corresponding acyl pyridinium intermediates, in turn, are amenable for C–C coupling under transition metal catalyst-free conditions, driven by the piezoelectric nature of barium titanate in a mechanochemical reaction setup. This approach shows a very general substrate scope, good functional group tolerance, and fair-to-excellent yields (50–92%, depending on the aniline derivative used) of benzophenone analogues. This acyl-intermediate-based method has been further applied to the synthesis of 3-benzoylchromones, attesting to its broad scope. Therefore, the described late-stage functionalization strategy, free of transition metals, represents progress in the scope of bioactive compound syntheses for medicinal chemistry.
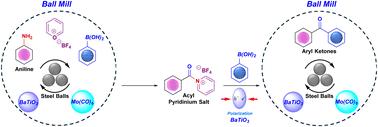
一锅Mo(CO)6催化苯胺通过原位吡啶重构转化为二苯甲酮
利用六羰基钼(Mo(CO)6)实现了苯胺类化合物的一锅式机械化学转化,避免了这种转化所固有的预功能化步骤。该反应利用苯胺中C(sp2) -NH2键的激活,通过原位形成吡啶盐,这一策略受到C - n键惰性的阻碍。在这个设想的方法中,使用四氟硼酸吡啶将苯胺转化为活性吡啶中间体,在Mo(CO)6存在下进行羰基化。相应的酰基吡啶中间体在机械化学反应装置中由钛酸钡的压电性质驱动,在无过渡金属催化剂的条件下易于C-C偶联。这种方法显示了一个非常广泛的底物范围,良好的官能团耐受性,以及相当优异的二苯甲酮类似物收率(50-92%,取决于所使用的苯胺衍生物)。这种酰基中间体为基础的合成方法已进一步应用于3-苯甲酰色酮的合成,证明了其广泛的应用范围。因此,所描述的后期功能化策略,不含过渡金属,代表了药物化学生物活性化合物合成范围的进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


