Tumor-resident probiotic Clostridium butyricum improves aPD-1 efficacy in colorectal cancer models by inhibiting IL-6-mediated immunosuppression
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Most colorectal cancer (CRC) patients do not respond to immune checkpoint blockade (ICB) therapy. Here, we identify Clostridium butyricum as a probiotic that boosts anti-PD-1 efficacy in CRC. In orthotopic allografts of microsatellite instability-high (MSI-H) and microsatellite stable (MSS) CRC, C. butyricum potentiates tumor suppressive effect of anti-PD-1, which is verified in AOM/DSS-induced CRC and germ-free mice. Single-cell RNA-seq reveals that C. butyricum activates cytotoxic CD8+ T lymphocytes (CTLs) and impairs tumor-associated macrophages (TAMs), especially in conjunction with anti-PD-1. Mechanistically, C. butyricum surface protein secD binds to CRC cell receptor glucose-regulated protein 78 (GRP78), which inactivates GRP78 and PI3K-AKT-NF-κB pathway, leading to reduced secretion of interleukin (IL)-6, an immunosuppressive cytokine that blunts CTLs and induces TAMs. Translational impact of C. butyricum in boosting anti-PD-1 efficacy is validated in huCD34+ humanized mice and autologous patient-derived CRC organoids-CTLs co-culture system. To summarize, C. butyricum is a promising adjuvant to augment ICB therapy.
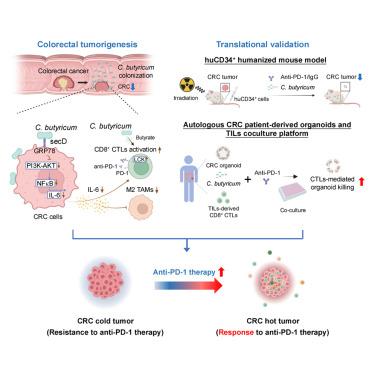
肿瘤常驻益生菌丁酸梭菌通过抑制il -6介导的免疫抑制提高aPD-1在结直肠癌模型中的疗效
大多数结直肠癌(CRC)患者对免疫检查点阻断(ICB)治疗没有反应。在这里,我们发现丁酸梭菌是一种益生菌,可以提高结直肠癌的抗pd -1功效。在微卫星不稳定-高(MSI-H)和微卫星稳定(MSS) CRC原位移植中,丁酸梭菌增强了抗pd -1的抑瘤作用,这在AOM/ dss诱导的CRC和无菌小鼠中得到了验证。单细胞RNA-seq显示,丁酸梭菌激活细胞毒性CD8+ T淋巴细胞(ctl)并损害肿瘤相关巨噬细胞(tam),特别是与抗pd -1联合使用。机制上,丁酸C. butyricum表面蛋白secD与CRC细胞受体葡萄糖调节蛋白78 (GRP78)结合,使GRP78和PI3K-AKT-NF-κB通路失活,导致白细胞介素(IL)-6分泌减少,IL -6是一种免疫抑制细胞因子,可钝化ctl并诱导tam。在huCD34+人源化小鼠和自体患者来源的CRC类器官- ctl共培养系统中,验证了丁酸梭菌增强抗pd -1功效的转化作用。综上所述,丁酸梭菌是一种很有前景的辅助ICB治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


