TLL1 knockdown attenuates prostate cancer progression by enhancing antitumor immunity
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Prostate cancer is one of the malignancies affecting men and contributes significantly to their increased mortality rates. Understanding the molecular mechanisms underlying the initiation and progression of prostate cancer is important for identifying potential drug targets. Here we showed that metalloproteinase TLL1 was positively associated with prostate cancer aggressiveness. Mechanistically, TLL1 promoted prostate cancer cells migration and metastasis through cleaving latent TGF-β1 to activate TGF-β signaling pathway. Moreover, LINC01179 interacted with Miz1 to attenuate TLL1 expression and LINC01179 impaired prostate cancer cell proliferation and migration ability by suppressing TLL1 expression to deactivate TGF-β signaling activity. Meanwhile, we observed that TLL1 increased the expression of PD-L1 by activating TGF-β signaling pathway and TLL1 depletion enhanced the antitumor efficacy by anti-PD-1 antibody via augmenting the infiltration proportions of CD8+ T cells in tumors. In addition, T cell-specific overexpression of TLL1 disrupted T cell development in the thymus. TLL1 overexpression in T cells accelerated RM-1 prostate tumor growth in mice by decreasing the infiltration of CD8+ T cells into tumors. Collectively, our results revealed that TLL1 may be a potential therapeutic target to alter prostate cancer progression.
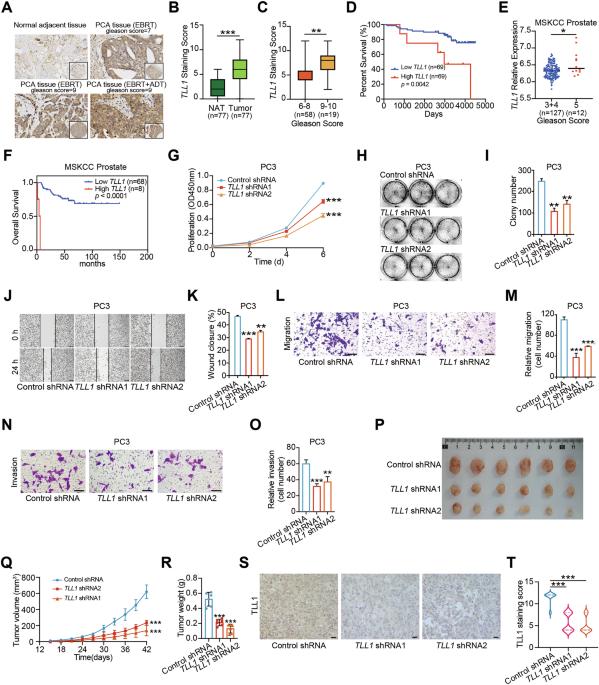
TLL1敲低可通过增强抗肿瘤免疫来减缓前列腺癌的进展。
前列腺癌是影响男性的恶性肿瘤之一,是导致男性死亡率上升的重要原因。了解前列腺癌发生和发展的分子机制对于确定潜在的药物靶点非常重要。本研究表明,金属蛋白酶TLL1与前列腺癌侵袭性呈正相关。在机制上,TLL1通过切割潜伏的TGF-β1激活TGF-β信号通路,促进前列腺癌细胞迁移转移。此外,LINC01179与Miz1相互作用,减弱TLL1的表达,LINC01179通过抑制TLL1的表达,使TGF-β信号活性失活,从而损害前列腺癌细胞的增殖和迁移能力。同时,我们观察到TLL1通过激活TGF-β信号通路增加PD-L1的表达,TLL1的缺失通过增加肿瘤中CD8+ T细胞的浸润比例来增强抗pd -1抗体的抗肿瘤效果。此外,TLL1的T细胞特异性过表达破坏了胸腺中T细胞的发育。TLL1在T细胞中的过表达通过减少CD8+ T细胞对肿瘤的浸润来加速小鼠RM-1前列腺肿瘤的生长。总之,我们的研究结果表明,TLL1可能是改变前列腺癌进展的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


