Novel flavonoid-magnesium complexes as inhibitors of plasma membrane calcium ATPase
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Plasma membrane calcium ATPases (PMCAs) are essential for regulating intracellular calcium (Ca2+) levels by extruding it from the cytosol. Improper regulation of these transporters is associated with numerous diseases, including neurological, cardiovascular, oncological, and metabolic problems, rendering them interesting targets for therapeutic intervention. However, there is a scarcity of specific tools to adjust PMCA activity. Flavonoids, a varied group of polyphenolic compounds with numerous biological effects, have been demonstrated to affect the function of several ATPases, including PMCAs.
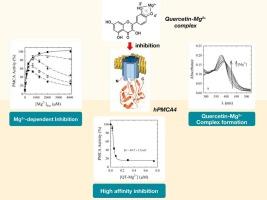
In this study, we investigated the inhibitory mechanism of quercetin on the human PMCA4 isoform (hPMCA4). Using UV–visible spectroscopy and ATPase activity assay, we identified a high-affinity inhibition mediated by a quercetin‑magnesium (Mg2+) complex with a Ki of 49.7 ± 1.5 nM. Functional and phosphorylation studies at different pHs suggest that quercetin affects PMCA activity through two inhibitory mechanisms: a high-affinity one mediated by the quercetin-Mg2+ complex and a low-affinity one mediated by the free flavonoid.
Analysis of the structure-activity relationship revealed that hydroxyl groups at positions 3′, 4′, and 3 are critical for complex formation and inhibitory potency. Specifically, the 3′ and 4′ hydroxyls are required to form the PMCA inhibitory complex. These findings demonstrate a novel mechanism of PMCA activity modulation involving flavonoid-Mg2+ complexes, which emerge as selective molecular tools capable of regulating Ca2+ transport. This knowledge provides new insights into designing PMCA inhibitors and exploring therapeutic strategies targeting diseases linked to calcium signalling dysfunction.
新型类黄酮-镁复合物作为质膜钙atp酶抑制剂
质膜钙atp酶(PMCAs)是必不可少的调节细胞内钙(Ca2+)水平通过挤出它从细胞质溶胶。这些转运蛋白的不当调控与许多疾病有关,包括神经、心血管、肿瘤和代谢问题,使它们成为治疗干预的有趣靶点。然而,缺乏特定的工具来调整PMCA活动。黄酮类化合物是一类具有多种生物效应的多酚类化合物,已被证明可以影响几种atp酶的功能,包括PMCAs。在本研究中,我们研究了槲皮素对人PMCA4亚型(hPMCA4)的抑制机制。通过紫外可见光谱和atp酶活性测定,我们发现槲皮素-镁(Mg2+)配合物介导的高亲和力抑制作用,Ki为49.7±1.5 nM。不同ph值下的功能和磷酸化研究表明,槲皮素通过两种抑制机制影响PMCA活性:槲皮素- mg2 +复合物介导的高亲和力抑制机制和游离类黄酮介导的低亲和力抑制机制。构效关系分析表明,3′、4′和3位的羟基对复合物的形成和抑制作用至关重要。具体来说,3 ‘和4 ’羟基是形成PMCA抑制复合物所必需的。这些发现证明了PMCA活性调节的新机制,涉及黄酮类- mg2 +复合物,这是一种能够调节Ca2+运输的选择性分子工具。这些知识为设计PMCA抑制剂和探索针对钙信号功能障碍相关疾病的治疗策略提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochimica et biophysica acta. Biomembranes
生物-生化与分子生物学
CiteScore
8.20
自引率
5.90%
发文量
175
审稿时长
2.3 months
期刊介绍:
BBA Biomembranes has its main focus on membrane structure, function and biomolecular organization, membrane proteins, receptors, channels and anchors, fluidity and composition, model membranes and liposomes, membrane surface studies and ligand interactions, transport studies, and membrane dynamics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


