Generation and evaluation of a novel PI3K-targeting gene therapy in the failing mouse heart and healthy sheep heart
IF 2.2
Journal of molecular and cellular cardiology plus
Pub Date : 2025-07-31
DOI:10.1016/j.jmccpl.2025.100478
引用次数: 0
Abstract
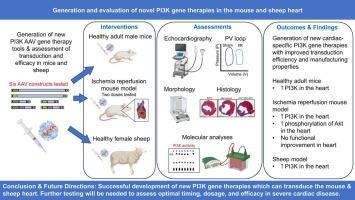
Heart failure (HF) remains a clinical challenge with cardiac dysfunction typically progressing even with treatment, and heart transplants only available to small numbers. We previously identified phosphoinositide 3-kinase (PI3K, p110α) as a master regulator of exercise-induced cardioprotection, and showed that gene therapy, incorporating a constitutively active form of PI3K (caPI3K) improved function of the failing mouse heart. However, this approach was not cardiac-specific and the gene therapy was challenging to manufacture. The aim of this study was to develop new PI3K-based gene therapies with more optimal properties for clinical translation. We generated and assessed adeno-associated viruses (AAV6) encoding various PI3K constructs, with different enhancers, promoters and transgene components in healthy adult male mice. The most promising AAV construct based on AAV expression, cardiac-specificity, and ease of manufacture contained a cardiac troponin T (cTnT) promoter together with a small region of the regulatory subunit of PI3K (iSH2), and an intron from the β-globin gene which enhances transcription (IVS2). This AAV (1 × 1012, 2 × 1012 vg) was administered to mice with myocardial ischemia/reperfusion injury (I/R: 1 h ischemia with reperfusion; AAV delivered 24 h post-I/R). Direct cardiac injections of PI3K-based AAVs were also performed in healthy adult female sheep. I/R mouse hearts treated with the AAV6-cTnT-IVS2-iSH2 displayed increased phosphorylation of Akt, but no improvement in cardiac function or structure was observed. AAV6-cTnT-IVS2-iSH2 successfully transduced healthy sheep hearts which increased endogenous PI3K catalytic activity. Further testing/optimization of the AAV (time of delivery and/or duration) will be required to assess the therapeutic potential of this approach.
一种新的pi3k靶向基因治疗衰竭小鼠心脏和健康绵羊心脏的产生和评估
心力衰竭(HF)仍然是一个临床挑战,即使治疗,心脏功能障碍通常也会恶化,心脏移植只适用于少数人。我们之前确定了磷酸肌肽3-激酶(PI3K, p110α)是运动诱导的心脏保护的主要调节因子,并表明基因治疗,包括组成活性形式的PI3K (caPI3K)改善了衰竭小鼠的心脏功能。然而,这种方法不是针对心脏的,而且这种基因疗法的制造具有挑战性。本研究的目的是开发新的基于pi3的基因疗法,具有更理想的临床转化特性。我们在健康成年雄性小鼠中生成并评估了编码多种PI3K构建体、具有不同增强子、启动子和转基因成分的腺相关病毒(AAV6)。基于AAV表达、心脏特异性和易于制造的最有希望的AAV构建物包含一个心脏肌钙蛋白T (cTnT)启动子、PI3K调控亚基的一个小区域(iSH2)和一个来自β-珠蛋白基因的内含子,该内含子可促进转录(IVS2)。将AAV (1 × 1012,2 × 1012vg)给予心肌缺血/再灌注损伤小鼠(I/R:缺血再灌注1 h;AAV在i /R后24小时交付)。在健康成年母羊中也进行了基于pi3的aav直接心脏注射。AAV6-cTnT-IVS2-iSH2处理的I/R小鼠心脏显示Akt磷酸化增加,但未观察到心脏功能或结构的改善。AAV6-cTnT-IVS2-iSH2成功转导了健康绵羊心脏,增加了内源性PI3K的催化活性。需要进一步测试/优化AAV(给药时间和/或持续时间)来评估该方法的治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of molecular and cellular cardiology plus
Cardiology and Cardiovascular Medicine
自引率
0.00%
发文量
0
审稿时长
31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


