Transcriptomic profiling reveals the adaptive mechanisms of Penaeus vannamei alkali-tolerant families under combined pH and alkalinity stress
IF 2.2
2区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology D-Genomics & Proteomics
Pub Date : 2025-08-05
DOI:10.1016/j.cbd.2025.101595
引用次数: 0
Abstract
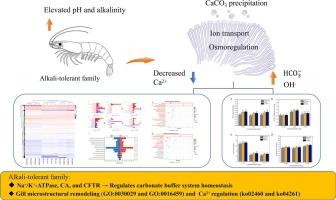
Soil salinization is an increasingly critical global issue for which fishery-based utilization has emerged as a promising mitigation strategy. Penaeus vannamei is an ideal species for aquaculture in saline-alkali waters; however, the differences in alkali tolerance among various families and their underlying mechanisms remain largely unexplored. In this study, alkali-tolerant families were identified through median lethal time (LT50) assays under high alkalinity and elevated pH. Comparative analyses with wild-type family revealed that alkali-tolerant families showed significant differences in energy metabolism and ion transport (Na+/K+-ATPase (NKA), carbonic anhydrase (CA), and cystic fibrosis transmembrane conductance regulator (CFTR)) Transcriptomic analysis showed that alkali-tolerant families had pathways related to cytoskeletal remodeling, including actin cytoskeleton organization (GO:0030029) and myosin complex (GO:0016459). KEGG analysis further revealed enrichment in cardiac muscle contraction (ko04260) and adrenergic signaling in cardiomyocytes (ko04261). We propose that CaCO3 precipitation reduces extracellular Ca2+ levels and disrupts the carbonate buffering system under high pH and alkalinity. In response, alkali-tolerant families mitigate pH and alkalinity stress by enhancing ion regulation and energy efficiency while simultaneously downregulating high-energy Ca2+ regulatory pathways and remodeling gill microstructures. Collectively, these findings provide novel insights into the alkali adaptation mechanisms of P. vannamei and support selective breeding strategies to improve stress resilience in saline-alkali aquaculture.
转录组学分析揭示了凡纳滨对虾耐碱家族在pH和碱度联合胁迫下的适应机制
土壤盐碱化是一个日益严重的全球问题,以渔业为基础的利用已成为一种有希望的缓解战略。凡纳滨对虾是盐碱水养殖的理想品种;然而,不同家族在碱耐受性方面的差异及其潜在机制在很大程度上仍未被探索。在本研究中,通过高碱度和高ph下的中位致死时间(LT50)测定鉴定了耐碱家族。与野生型家族的比较分析显示,耐碱家族在能量代谢和离子转运(Na+/K+- atp酶(NKA)、碳酸酐酶(CA)、转录组学分析显示,耐碱家族具有与细胞骨架重塑相关的途径,包括肌动蛋白细胞骨架组织(GO:0030029)和肌球蛋白复合物(GO:0016459)。KEGG分析进一步显示心肌收缩(ko04260)和心肌细胞的肾上腺素能信号(ko04261)富集。我们认为CaCO3沉淀降低了细胞外Ca2+水平,并破坏了高pH和高碱度下的碳酸盐缓冲系统。因此,耐碱家族通过增强离子调节和能量效率来缓解pH和碱度胁迫,同时下调高能Ca2+调节途径和重塑鳃微结构。综上所述,这些发现为瓦纳梅对虾的碱适应机制提供了新的见解,并为提高盐碱水产养殖的胁迫适应能力的选择性育种策略提供了支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.10
自引率
3.30%
发文量
69
审稿时长
33 days
期刊介绍:
Comparative Biochemistry & Physiology (CBP) publishes papers in comparative, environmental and evolutionary physiology.
Part D: Genomics and Proteomics (CBPD), focuses on “omics” approaches to physiology, including comparative and functional genomics, metagenomics, transcriptomics, proteomics, metabolomics, and lipidomics. Most studies employ “omics” and/or system biology to test specific hypotheses about molecular and biochemical mechanisms underlying physiological responses to the environment. We encourage papers that address fundamental questions in comparative physiology and biochemistry rather than studies with a focus that is purely technical, methodological or descriptive in nature.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


