Brain Latent Progression: Individual-based spatiotemporal disease progression on 3D Brain MRIs via latent diffusion
IF 11.8
1区 医学
Q1 COMPUTER SCIENCE, ARTIFICIAL INTELLIGENCE
引用次数: 0
Abstract
The growing availability of longitudinal Magnetic Resonance Imaging (MRI) datasets has facilitated Artificial Intelligence (AI)-driven modeling of disease progression, making it possible to predict future medical scans for individual patients. However, despite significant advancements in AI, current methods continue to face challenges including achieving patient-specific individualization, ensuring spatiotemporal consistency, efficiently utilizing longitudinal data, and managing the substantial memory demands of 3D scans. To address these challenges, we propose Brain Latent Progression (BrLP), a novel spatiotemporal model designed to predict individual-level disease progression in 3D brain MRIs. The key contributions in BrLP are fourfold: (i) it operates in a small latent space, mitigating the computational challenges posed by high-dimensional imaging data; (ii) it explicitly integrates subject metadata to enhance the individualization of predictions; (iii) it incorporates prior knowledge of disease dynamics through an auxiliary model, facilitating the integration of longitudinal data; and (iv) it introduces the Latent Average Stabilization (LAS) algorithm, which (a) enforces spatiotemporal consistency in the predicted progression at inference time and (b) allows us to derive a measure of the uncertainty for the prediction at the global and voxel level. We train and evaluate BrLP on 11,730 T1-weighted (T1w) brain MRIs from 2,805 subjects and validate its generalizability on an external test set comprising 2,257 MRIs from 962 subjects. Our experiments compare BrLP-generated MRI scans with real follow-up MRIs, demonstrating state-of-the-art accuracy compared to existing methods. The code is publicly available at: https://github.com/LemuelPuglisi/BrLP.
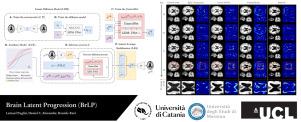
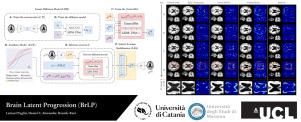
脑潜伏进展:通过潜伏扩散在三维脑mri上显示基于个体的时空疾病进展
纵向磁共振成像(MRI)数据集的日益可用性促进了人工智能(AI)驱动的疾病进展建模,使预测个体患者未来的医学扫描成为可能。然而,尽管人工智能取得了重大进展,但目前的方法仍然面临挑战,包括实现针对患者的个性化、确保时空一致性、有效利用纵向数据以及管理3D扫描的大量内存需求。为了解决这些挑战,我们提出了脑潜伏进展(BrLP),这是一种新的时空模型,旨在通过3D脑mri预测个体水平的疾病进展。BrLP的主要贡献有四个方面:(i)它在很小的潜在空间中运行,减轻了高维成像数据带来的计算挑战;(ii)明确整合主体元数据,增强预测的个性化;(iii)通过辅助模型纳入疾病动力学的先验知识,促进纵向数据的整合;(iv)它引入了潜在平均稳定(LAS)算法,该算法(a)在推理时间强制预测进程的时空一致性,(b)允许我们在全局和体素水平上得出预测的不确定性度量。我们在来自2805名受试者的11730张t1加权(T1w)脑mri上训练和评估BrLP,并在包含来自962名受试者的2257张mri的外部测试集上验证其泛化性。我们的实验将brlp生成的MRI扫描与真实的后续MRI扫描进行了比较,与现有方法相比,展示了最先进的准确性。该代码可在https://github.com/LemuelPuglisi/BrLP公开获取。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Medical image analysis
工程技术-工程:生物医学
CiteScore
22.10
自引率
6.40%
发文量
309
审稿时长
6.6 months
期刊介绍:
Medical Image Analysis serves as a platform for sharing new research findings in the realm of medical and biological image analysis, with a focus on applications of computer vision, virtual reality, and robotics to biomedical imaging challenges. The journal prioritizes the publication of high-quality, original papers contributing to the fundamental science of processing, analyzing, and utilizing medical and biological images. It welcomes approaches utilizing biomedical image datasets across all spatial scales, from molecular/cellular imaging to tissue/organ imaging.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


