Ectopic laminin α2 accumulation in the glomerular basement membrane exacerbates podocyte injury in Alport syndrome
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-08-05
DOI:10.1016/j.bbadis.2025.168008
引用次数: 0
Abstract
Alport syndrome is a hereditary disease caused by mutations in Col4a3, Col4a4, and Col4a5, which encode the type IV collagen α3, α4, and α5 chains, respectively. Alport glomerular basement membrane (GBM), which predominantly consists of collagen α1α2α1 (IV) heterotrimers, provides less biomechanical strength than normal GBM with collagen α3α4α5 (IV) heterotrimers. In addition to type IV collagen abnormalities, laminin dysregulation is observed in Alport GBM. In this study, we aimed to investigate the mechanisms underlying laminin dysregulation in Alport GBM and their roles in disease progression using primary podocytes and Col4a4-deficient mice on DBA/2 background. Histological analysis of Col4a4-deficient mice revealed that ectopic laminin α2 deposition in GBM during postnatal nephrogenesis, followed by re-expression of laminin α1 and decreased expression of nephrin. The analysis of primary podocytes indicated that podocytes on low substrate stiffness overexpressed laminin α2. Moreover, podocytes cultured on laminin-α2β1γ1 exhibited higher laminin α1 levels and lower nephrin levels than those cultured on laminin-α5β2γ1. Cell adhesion assays showed that ectopic laminin α2 deposition in GBM may cause defective podocyte–GBM adhesion, leading to podocyte depletion. Overall, these findings suggest that insufficient GBM strength increases the mechanical stress on podocytes via daily transcapillary filtration pressures, resulting in ectopic laminin α2 deposition in GBM, which contributes to defective podocyte–GBM adhesion, GBM abnormalities, and podocyte injury in Alport syndrome.
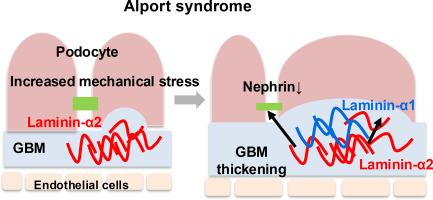
肾小球基底膜异位层粘连蛋白α2积聚加重Alport综合征足细胞损伤。
Alport综合征是一种由编码IV型胶原α3、α4、α5链的Col4a3、Col4a4、Col4a5突变引起的遗传性疾病。肾小球基底膜(GBM)主要由胶原α1α2α1 (IV)三聚体组成,其生物力学强度低于含有胶原α3α4α5 (IV)三聚体的正常肾小球基底膜。除了IV型胶原异常外,在Alport GBM中观察到层粘连蛋白失调。在这项研究中,我们旨在研究Alport GBM中层粘连蛋白失调的机制及其在DBA/2背景下原代足细胞和col4a4缺陷小鼠疾病进展中的作用。col4a4缺陷小鼠的组织学分析显示,在出生后肾形成过程中,层粘连蛋白α2异位沉积在GBM中,随后层粘连蛋白α1重新表达,肾素表达降低。原代足细胞分析表明,低底物刚度足细胞过表达层粘连蛋白α2。此外,与层粘连蛋白-α2β1γ1培养的足细胞相比,层粘连蛋白-α5β2γ1培养的足细胞表现出较高的层粘连蛋白α1水平和较低的肾素水平。细胞粘附实验表明,异位层粘连蛋白α2沉积在GBM中可能导致足细胞与GBM的粘附缺陷,导致足细胞耗损。总之,这些研究结果表明,GBM强度不足增加了足细胞通过每日经毛细血管滤过压力所承受的机械应力,导致GBM中层粘连蛋白α2异位沉积,从而导致Alport综合征足细胞-GBM粘附缺陷、GBM异常和足细胞损伤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


